Abstract
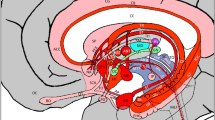
The hypothalamus and its subdivisions are involved in many neuropsychiatric conditions such as affective disorders, schizophrenia, or narcolepsy, but parcellations of hypothalamic subnuclei have hitherto been feasible only with histological techniques in postmortem brains. In an attempt to map subdivisions of the hypothalamus in vivo, we analyzed the directionality information from high-resolution diffusion-weighted magnetic resonance images of healthy volunteers. We acquired T1-weighted and diffusion-weighted scans in ten healthy subjects at 3 T. In the T1-weighted images, we manually delineated an individual mask of the hypothalamus in each subject and computed in the co-registered diffusion-weighted images the similarity of the principal diffusion direction for each pair of mask voxels. By clustering the similarity matrix into three regions with a k-means algorithm, we obtained an anatomically coherent arrangement of subdivisions across hemispheres and subjects. In each hypothalamus mask, we found an anterior region with dorsoventral principal diffusion direction, a posteromedial region with rostro-caudal direction, and a lateral region with mediolateral direction. A comparative analysis with microstructural hypothalamus parcellations from the literature reveals that each of these regions corresponds to a specific group of hypothalamic subnuclei as defined in postmortem brains. This is to our best knowledge the first in vivo study that attempts a delineation of hypothalamic subdivisions by clustering diffusion-weighted magnetic resonance imaging data. When applied in a larger sample of neuropsychiatric patients, a structural analysis of hypothalamic subnuclei should contribute to a better understanding of the pathogenesis of neuropsychiatric conditions such as affective disorders.





Similar content being viewed by others
References
Alsop DC (1997) The sensitivity of low flip angle RARE imaging. Magn Reson Med 37:176–184
Bao AM, Meynen G, Swaab DF (2008) The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev 57:531–553
Baroncini M, Jissendi P, Balland E, Besson P, Pruvo JP, Francke JP, Dewailly D, Blond S, Prevot V (2012) MRI atlas of the human hypothalamus. Neuroimage 59:168–180
Baumann B, Bogerts B (2001) Neuroanatomical studies on bipolar disorder. Br J Psychiatry 178:S142–S147
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 15:435–455
Bernstein HG, Keilhoff G, Steiner J, Dobrowolny H, Bogerts B (2010) The hypothalamus in schizophrenia research: no longer a wallflower existence. Open Neuroendocrinol J 3:59–67
Bernstein HG, Klix M, Dobrowolny H, Brisch R, Steiner J, Bielau H, Gos T, Bogerts B (2012) A postmortem assessment of mammillary body volume, neuronal number and densities, and fornix volume in subjects with mood disorders. Eur Arch Psychiatry Clin Neurosci 262:637–646
Besseling RMH, Jansen JFA, Overvliet GM, Vaessen MJ, Braakman HMH, Hofman PAM, Aldenkamp AP, Backes WH (2012) Tract specific reproducibility of tractography based morphology and diffusion metrics. PLoS One 7:e34125
Bielau H, Trübner K, Krell D, Agelink MW, Bernstein HG, Stauch R, Mawrin C, Danos P, Gerhard L, Bogerts B, Baumann B (2005) Volume deficits of subcortical nuclei in mood disorders: a postmortem study. Eur Arch Psychiatry Clin Neurosci 255:401–412
Braak H, Braak E (1987) The hypothalamus of the human adult: chiasmatic region. Anat Embryol (Berl) 175:315–330
Braak H, Braak E (1992) Anatomy of the human hypothalamus (chiasmatic and tuberal region). In: Swaab DF, Hofman MA, Mirmiran M, Ravid R, Van Leeuwen FW (eds) Progress in brain research. Elsevier, Amsterdam, pp 3–16
Busse S, Bernstein HG, Busse M, Bielau H, Brisch R, Mawrin C, Müller S, Sarnyai Z, Gos T, Bogerts B, Steiner J (2012) Reduced density of hypothalamic VGF-immunoreactive neurons in schizophrenia: a potential link to impaired growth factor signaling and energy homeostasis. Eur Arch Psychiatry Clin Neurosci 262:365–374
Crosby EC, Woodburne RT (1939) The comparative anatomy of the preoptic area and the hypothalamus. Res Publ Assoc Res Nerv Ment Dis 20:52–169
Doran M, Hajnal JV, Van Bruggen N, King MD, Young IR, Bydder GM (1990) Normal and abnormal white matter tracts shown by MR imaging using directional diffusion weighted sequences. J Comput Assist Tomogr 14:865–873
Draganski B, Geisler P, Hajak G, Schuierer G, Bogdahn U, Winkler J, May A (2002) Hypothalamic gray matter changes in narcoleptic patients. Nat Med 8:1186–1188
Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213:93–118
Dupont RM, Jernigan TL, Heindel W, Butters N, Shafer K, Wilson T, Hesselink J, Gillin JC (1995) Magnetic resonance imaging and mood disorders: localization of white matter and other subcortical abnormalities. Arch Gen Psychiatry 52:747–755
Georgiadis JR, Farrell MJ, Boessen R, Denton DA, Gavrilescu M, Kortekaas R, Renken RJ, Hoogduin JM, Egan GF (2010) Dynamic subcortical blood flow during male sexual activity with ecological validity: a perfusion fMRI study. Neuroimage 50:208–216
Geyer S, Weiss M, Reimann K, Lohmann G, Turner R (2011) Microstructural parcellation of the human cerebral cortex: from Brodmann’s post-mortem map to in vivo mapping with high-field magnetic resonance imaging. Front Hum Neurosci 5:19
Goldstein JM, Seidman LJ, Makris N, Ahern T, O’Brien LM, Caviness VS, Kennedy DN, Faraone SV, Tsuang MT (2007) Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry 61:935–945
Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A (2002) Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 47:1202–1210
Hegerl U, Wilk K, Olbrich S, Schoenknecht P, Sander C (2012) Hyperstable regulation of vigilance in patients with major depressive disorder. World J Biol Psychiatry 13:436–446
Hofman MA, Swaab DF (1992) The human hypothalamus: comparative morphometry and photoperiodic influences. In: Swaab DF, Hofman MA, Mirmiran M, Ravid R, Van Leeuwen FW (eds) Progress in brain research. Elsevier, Amsterdam, pp 133–149
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62:782–790
Johansen-Berg H, Behrens TEJ, Robson MD, Drobnjak I, Rushworth MFS, Brady JM, Smith SM, Higham DJ, Matthews PM (2004) Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA 101:13335–13340
Johansen-Berg H, Behrens TEJ, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM (2005) Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex 15:31–39
Jones DK, Knösche TR, Turner R (2012) White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. doi:10.1016/j.neuroimage.2012.06.081
Klein JC, Behrens TEJ, Robson MD, Mackay CE, Higham DJ, Johansen-Berg H (2007) Connectivity-based parcellation of human cortex using diffusion MRI: establishing reproducibility, validity and observer independence in BA 44/45 and SMA/pre-SMA. Neuroimage 34:204–211
Koutcherov Y, Mai JK, Paxinos G (2003) Hypothalamus of the human fetus. J Chem Neuroanat 26:253–270
Kwon HG, Byun WM, Ahn SH, Son SM, Jang SH (2011) The anatomical characteristics of the stria terminalis in the human brain: a diffusion tensor tractography study. Neurosci Lett 500:99–102
Le Bihan D (2003) Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4:469–480
Le Gros Clark WE (1936) The topography and homologies of the hypothalamic nuclei in man. J Anat 70:203–214
Le Gros Clark WE (1938) Morphological aspects of the hypothalamus. In: Le Gros Clark WE, Beattie J, Riddoch G, Dott NM (eds) The hypothalamus: morphological, functional, clinical and surgical aspects. Oliver and Boyd, Edinburgh, pp 1–68
Le Roux P, Hinks RS (1993) Stabilization of echo amplitudes in FSE sequences. Magn Reson Med 30:183–190
Lemaire JJ, Frew AJ, McArthur D, Gorgulho AA, Alger JR, Salomon N, Chen C, Behnke EJ, De Salles AAF (2011) White matter connectivity of human hypothalamus. Brain Res 1371:43–64
Li M, He HG, Shi W, Li J, Lv B, Wang CH, Miao QW, Wang ZC, Wang NL, Walter M, Sabel BA (2012) Quantification of the human lateral geniculate nucleus in vivo using MR imaging based on morphometry: volume loss with age. Am J Neuroradiol 33:915–921
Luppino G, Matelli M, Camarda R, Rizzolatti G (1993) Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol 338:114–140
Mai JK, Paxinos G, Voss T (2008) Atlas of the human brain, 3rd edn. Elsevier, Academic Press, Amsterdam
Marques JP, Kober T, Krueger G, van der Zwaag W, van de Moortele PF, Gruetter R (2010) MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49:1271–1281
Matelli M, Luppino G (1996) Thalamic input to mesial and superior area 6 in the macaque monkey. J Comp Neurol 372:59–87
Moseley ME, Cohen Y, Kucharczyk J, Mintorovitch J, Asgari HS, Wendland MF, Tsuruda J, Norman D (1990) Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology 176:439–445
Mugler JP, Brookeman JR (1990) Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 15:152–157
Nanetti L, Cerliani L, Gazzola V, Renken R, Keysers C (2009) Group analyses of connectivity-based cortical parcellation using repeated k-means clustering. Neuroimage 47:1666–1677
Nieuwenhuys R, Voogd J, van Huijzen C (2008) The human central nervous system, 4th edn. Springer, Berlin
Pinilla BP (2009) Auswirkungen der unipolar depressiven Störung auf strukturelle Gehirnveränderungen in der Voxel-based-NMR-Morphometry und auf “hippocampusspezifische” kognitive Leistungen. Dissertation, Charité, Berlin
Price JL, Drevets WC (2010) Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216
Reese TG, Heid O, Weisskoff RM, Wedeen VJ (2003) Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med 49:177–182
Rizzolatti G, Luppino G, Matelli M (1996) The classic supplementary motor area is formed by two independent areas. In: Lüders HO (ed) Supplementary sensorimotor area. Lippincott-Raven, Philadelphia, pp 45–56
Saeki N, Kansaku K, Higuchi Y, Kawano K, Iijima T, Inoue N, Yamaura A (2001) Demonstration of the post commissural fibres of the fornix in short-inversion time inversion-recovery imaging on a high-field system. Neuroradiology 43:547–550
Saper CB (2012) Hypothalamus. In: Mai JK, Paxinos G (eds) The human nervous system, 3rd edn. Elsevier, Academic Press, Amsterdam, pp 548–583
Schindler S, Geyer S, Strauß M, Anwander A, Hegerl U, Turner R, Schönknecht P (2012) Structural studies of the hypothalamus and its nuclei in mood disorders. Psychiatry Res 201:1–9
Schmidt FM, Brügel M, Kratzsch J, Strauß M, Sander C, Baum P, Thiery J, Hegerl U, Schönknecht P (2010) Cerebrospinal fluid hypocretin-1 (orexin A) levels in mania compared to unipolar depression and healthy controls. Neurosci Lett 483:20–22
Schmidt FM, Arendt E, Steinmetzer A, Bruegel M, Kratzsch J, Strauß M, Baum P, Hegerl U, Schönknecht P (2011) CSF-hypocretin-1 levels in patients with major depressive disorder compared to healthy controls. Psychiatry Res 190:240–243
Schoenknecht P, Olbrich S, Sander C, Spindler P, Hegerl U (2010) Treatment of acute mania with modafinil monotherapy. Biol Psychiatry 67:e55–e57
Solano-Castiella E, Anwander A, Lohmann G, Weiss M, Docherty C, Geyer S, Reimer E, Friederici AD, Turner R (2010) Diffusion tensor imaging segments the human amygdala in vivo. Neuroimage 49:2958–2965
Stephan H, Frahm H, Baron G (1981) New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol (Basel) 35:1–29
Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain. 3-Dimensional proportional system: an approach to cerebral imaging. Thieme, Stuttgart
Toni R, Malaguti A, Benfenati F, Martini L (2004) The human hypothalamus: a morpho-functional perspective. J Endocrinol Invest 27:73–94
Walter M, Bermpohl F, Mouras H, Schiltz K, Tempelmann C, Rotte M, Heinze HJ, Bogerts B, Northoff G (2008) Distinguishing specific sexual and general emotional effects in fMRI: subcortical and cortical arousal during erotic picture viewing. Neuroimage 40:1482–1494
Wiegell MR, Tuch DS, Larsson HBW, Wedeen VJ (2003) Automatic segmentation of thalamic nuclei from diffusion tensor magnetic resonance imaging. Neuroimage 19:391–401
Young JK, Stanton GB (1994) A three-dimensional reconstruction of the human hypothalamus. Brain Res Bull 35:323–327
Acknowledgments
Part of this work was supported by the FET project CONNECT of the European Union (http://www.brain-connect.eu).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schönknecht, P., Anwander, A., Petzold, F. et al. Diffusion imaging-based subdivision of the human hypothalamus: a magnetic resonance study with clinical implications. Eur Arch Psychiatry Clin Neurosci 263, 497–508 (2013). https://doi.org/10.1007/s00406-012-0389-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-012-0389-5