Abstract
Background
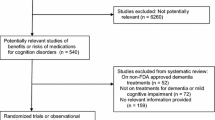
Individual randomized clinical trials (RCTs) with cholinesterase inhibitors (ChEIs) aiming to delay the progression from mild cognitive impairment (MCI) to Alzheimer’s disease (AD) have not found significant benefit of their use for this purpose. The objective of this study is to meta-analyze the RCTs conducted with ChEIs in order to assess whether pooled analysis could show the benefit of these drugs in delaying the progression from MCI to AD.
Methods
We searched for references of published and unpublished studies on electronic databases (Medline, Embase, Web of Science, and Clinical Trial Database Registry, particularly the Clinicaltrials.gov—http://www.clinicaltrials.gov). We retrieved 173 references, which yielded three references for data extraction. A total of 3.574 subjects from four RCTs were included in the meta-analysis. Among 1,784 subjects allocated in the ChEI-treatment group, 275 (15.4%) progressed to AD/dementia, as opposed to 366 (20.4%) out of 1,790 subjects in the placebo group. The relative risk (RR) for progression to AD/dementia in the ChEI-treated group was 0.75 [CI95% 0.66–0.87], z = −3.89, P < 0.001. The patients on the ChEI group had a significantly higher all-cause dropout risk than the patients on the placebo group (RR = 1.36 CI95% [1.24–1.49]; z = 6.59, P < 0.001). The RR for serious adverse events (SAE) in the ChEI-treated group showed no significantly statistical difference from the placebo group (RR = 0.95 [CI95% 0.83–1.09], z = −0.72, P = 0.47). The subjects in the ChEI-treated group had a marginally, non-significant, higher risk of death due to any cause than those in the placebo-treated group (RR = 1.04, CI95% 0.63–1.70, z = 0.16, P = 0.86).
Conclusion
The long-term use of ChEIs in subjects with MCI may attenuate the risk of progression to AD/dementia. This finding may have a significant impact on public health and pharmaco-economic policies.





Similar content being viewed by others
References
[No author listed] (2004) Efficacy and safety of rivastigmine in patients with mild cognitive impairment. NCT00134953. Available on-line on http://www.clinicaltrials.gov/ct2/show/NCT00134953. Accessed 27 Oct 2008
[No author’s listed]. (2007) A randomized double blind parallel, placebo-controlled trial to examine the efficacy of oral donepezil (5 mg qd for 6 weeks) after single dose and steady state therapy (2 and 6 weeks) in subjects with mild cognitive impairment. NCT00483028. Available on-line on http://clinicaltrials.gov/ct2/show/NCT00483028. Accessed 27 Oct 2008
[No authors listed] (2004) Cognitive enhancers explored with PET imaging. NCT 00042172. Available on-line http://clinicaltrials.gov/ct2/show/NCT00042172. Accessed 27 Oct 2008
Ames D, Petersen RC, Knopman DS, Visser PJ, Brodaty H, Gauthier S (2006) Is mild cognitive impairment a clinically useful concept? Int Psychogeriatr 18:393–414
Anita M (2007) A one-year, multicenter, randomized, double-blind, placebo controlled evaluation of the efficacy and safety of donepezil hydrochloride in subjects with mild cognitive impairment. NCT00293176. Available on line http://clinicaltrials.gov/ct2/show/NCT00293176. Accessed 27 Oct 2008
Bäckman L, Small BJ, Fratiglioni L (2001) Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain 124:96–102
Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer’s disease. Alzheimers Dement 3:186–191
Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P (1992) Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci USA 89:10075–10078
Cummings JL, Doody R, Clark C (2007) Disease-modifying therapies for Alzheimer disease: challenges to early intervention. Neurology 69:1622–1634
Diniz BS, Pinto JA Jr, Forlenza OV (2008) Do CSF total tau, phosphorylated tau, and beta-amyloid 42 help to predict progression of mild cognitive impairment to Alzheimer’s disease? A systematic review and meta-analysis of the literature. World J Biol Psychiatry 9:172–182
Doody R, Geldmacher D, Gordon B, Perdomo CA, Pratt RD, Donepezil Study Group (2001) Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol 58:427–433
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro OJ, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P (2007) Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6:734–746
Farias GG, Godoy JA, Hernandez F, Avila J, Fisher A, Inestrosa NC (2004) M1 muscarinic receptor activation protects neurons from beta-amyloid toxicity: a role for Wnt signaling pathway. Neurobiol Dis 17:337–348
Feldman HH, Ferris S, Winblad B, Sfikas N, Mancione L, He Y, Tekin S, Burns A, Cummings J, del Ser T, Inzitari D, Orgogozo JM, Sauer H, Scheltens P, Scarpini E, Herrmann N, Farlow M, Potkin S, Charles HC, Fox NC, Lane R (2007) Effect of rivastigmine on delay to diagnosis of Alzheimer’s disease from mild cognitive impairment: the InDDEx study. Lancet Neurol 6:501–512
Forlenza OV, Chiu E (2008) Mild cognitive impairment: a concept ready to move on? Curr Opin Psychiatry 21:529–532
Forlenza OV, Spink JM, Dayanandan R, Anderton BH, Olesen OF, Lovestone S (2000) Muscarinic agonists reduce tau phosphorylation in non-neuronal cells via GSK-3beta inhibition and in neurons. J Neural Transm 107:1201–1212
Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L (2006) Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5:228–234
Hashimoto M, Kazui H, Matsumoto K, Nakano Y, Yasuda M, Mori E (2005) Does donepezil treatment slow the progression of hippocampal atrophy in patients with Alzheimer’s disease? Am J Psychiatry 162:676–682
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104:1433–1439
Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary. Controlled Clin Trials 17:1–12
Koontz J, Baskys A (2005) Effects of galantamine on working memory and global functioning in patients with mild cognitive impairment: a double-blind placebo-controlled study. Am J Alzheimers Dis Other Demen 20:295–302
Korf ESC, Wahlund LO, Visser PJ, Scheltens P (2004) Medial temporal lobe atrophy on MRI predicts dementia in patients with mild cognitive impairment. Neurology 63:94–100
Lanctôt KL, Herrmann N, Yau KK, Khan LR, Liu BA, LouLou MM, Einarson TR (2003) Efficacy and safety of cholinesterase inhibitors in Alzheimer’s disease: a meta-analysis. CMAJ 169:557–564
Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C (2008) Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 258:124–128
López-Pousa S, Turon-Estrada A, Garre-Olmo J, Pericot-Nierga I, Lozano-Gallego M, Vilalta-Franch M, Hernández-Ferràndiz M, Morante-Muñoz V, Isern-Vila A, Gelada-Batlle E, Majó-Llopart J (2005) Differential efficacy of treatment with cholinesterase inhibitors in patients with mild and moderate Alzheimer’s disease over a 6-month period. Dement Geriatr Cogn Disord 19:189–195
Mahley RW, Weisgraber KH, Huang Y (2006) Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci USA 103:5644–5651
Mendes CT, Mury FB, de Sá Moreira E, Alberto FL, Forlenza OV, Dias-Neto E, Gattaz WF (2008) Lithium reduces Gsk3b mRNA levels: implications for Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci [Epub ahead of print]
National Institute for Clinical Excellence (NICE) (2004) Management of depression in primary and secondary care. The British Psychological Society and Gaskel. Leicester
Nitsch RM, Slack BE, Wurtman RJ, Growdon JH (1992) Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 28:304–307
Petersen RC (2006) Conversion. Neurology 67(Suppl. 3):s12–s13
Petersen RC (2007) MCI treatment trials: failure or not? Lancet Neurol 6:473–475
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308
Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ, Alzheimer’s Disease Cooperative Study Group (2005) Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 352:2379–2388
Pirttila T, Wilcock G, Truyen L, Damaraju CV (2004) Long-term efficacy and safety of galantamine in patients with mild-to-moderate Alzheimer’s disease: multicenter trial. Eur J Neurol 11:734–741
Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S (2008) Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70:440–448
Ritchie K, Artero S, Touchon J (2001) Classification criteria for mild cognitive impairment: a population-based validation study. Neurology 56:37–42
Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT (1998) A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease: Donepezil Study Group. Neurology 50:136–145
Rösler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, Stähelin HB, Hartman R, Gharabawi M (1999) Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ 318:633–638
Sabbagh MN, Farlow MR, Relkin N, Beach TG (2006) Do cholinergic therapies have disease-modifying effects in Alzheimer’s disease? Alzheimers Dement 2:118–125
Salloway S, Ferris S, Kluger A, Goldman R, Griesing T, Kumar D, Richardson S (2004) Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 63:651–657
Saykin AJ, Wishart HA, Rabin LA, Flashman LA, McHugh TL, Mamourian AC, Santulli RB (2004) Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain 127:1574–1583
Small GW, Kaufer D, Mendiondo MS, Quarg P, Spiegel R (2005) Cognitive performance in Alzheimer’s disease patients receiving rivastigmine for up to 5 years. Int J Clin Pract 59:473–477
Vellas B, Andrieu S, Sampaio C, Coley N, Wilcock G, for the European Task Force Group (2008) Endpoints for trials in Alzheimer’s disease: a European task force consensus. Lancet Neurol 7:436–450
Visser PJ, Sheltens P, Verhey FRJ (2005) Do MCI criteria in drug trials accurately identify subjects with pre-dementia Alzheimer’s disease? J Neurol Neurosurg Psychiatry 76: 1348–1354
Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR Jr (2008) MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology 70: 512–520
Wilcock GK, Lilienfeld S, Gaens E (2000) Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ 321:1445–1449
Wilkinson DG, Passmore AP, Bullock R, Hopker SW, Smith R, Potocnik FC, Maud CM, Engelbrecht I, Hock C, Ieni JR, Bahra RS (2002) A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer’s disease. Int J Clin Pract 56:441–446
Wimo A, Winblad B, Jönsson L (2007) An estimate of the total worldwide societal costs of dementia in 2005. Alzheimers Dement 3:81–91
Winblad B, Gauthier S, Scinto L, Feldman H, Wilcock GK, Truyen L, Mayorga AJ, Wang D, Brashear HR, Nye JS (2008) Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 70:2024–2035
Wolf BA, Wertkin AM, Jolly YC, Yasuda RP, Wolfe BB, Konrad RJ, Manning D, Ravi S, Williamson JR, Lee VM (1995) Muscarinic regulation of Alzheimer’s disease amyloid precursor protein secretion and amyloid beta-protein production in human neuronal NT2N cells. J Biol Chem 270:4916–4922
Conflict of interest
Dr. Gattaz has received research support and speakership honoraria from AstraZeneca, Bristol Myers-Squibb, Eli Lilly, and Janssen-Cilag.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Diniz, B.S., Pinto, J.A., Gonzaga, M.L.C. et al. To treat or not to treat? A meta-analysis of the use of cholinesterase inhibitors in mild cognitive impairment for delaying progression to Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci 259, 248–256 (2009). https://doi.org/10.1007/s00406-008-0864-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-008-0864-1