Abstract
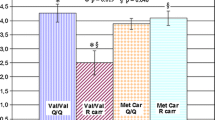
The synaptosomal-associated protein of 25 kDa (SNAP-25) is part of the soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment receptor (SNARE), which mediates synaptic neurotransmission. In earlier studies a possible involvement of this protein in schizophrenia has been shown. As neurocognitive impairment is a core feature in the pathology of schizophrenia and considered to be a putative endophenotype according to genetic studies we investigated the influences of different SNAP-25 polymorphisms on neuropsychological test results before and during treatment with atypical antipsychotics. A total of 104 schizophrenic patients treated with atypical antipsychotics were genotyped for three different polymorphisms of the SNAP-25 gene (MnlI, TaiI and DdeI in the 3′-UTR). Cognitive function was assessed at baseline, week 4 or 6 and week 8 or 12. Results of individual neuropsychological tests were assigned to six cognitive domains (reaction time and quality; executive function; working, verbal and visual memory) and a general cognitive index. The MnlI and TaiI polymorphisms showed no associations to deficits on neuropsychological test results. In contrast, we observed a significant relation between the DdeI polymorphism of the SNAP-25 gene and cognitive dysfunctions. Homozygote T/T allele carriers of the DdeI polymorphism showed significant better neuropsychological test results in cognitive domains verbal memory and executive functions than those with the combined T/C and C/C genotypes (P < 0.01) at all three time points, but no differences in response to treatment with atypical antipsychotics. Additionally, TT carriers exhibited significantly better results in a general cognitive index (P < 0.05). As we observed an association between the DdeI polymorphism of the SNAP-25 gene and cognitive dysfunctions of schizophrenic patients our finding suggests that the SNAP-25 gene could play a role in the pathophysiology of neurocognitive dysfunctions in schizophrenia but is not predictive for treatment response with atypical antipsychotics.


Similar content being viewed by others
References
Albus M, Hubmann W, Mohr F, Hecht S, Hinterberger-Weber P, Seitz NN, Kuchenhoff H (2006) Neurocognitive functioning in patients with first-episode schizophrenia: results of a prospective 5-year follow-up study. Eur Arch Psychiatry Clin Neurosci 256:442–451
Bajjalieh SM, Scheller RH (1995) The biochemistry of neurotransmitter secretion. J Biol Chem 270:1971–1974
Barr AM, Young CE, Phillips AG, Honer WG (2006) Selective effects of typical antipsychotic drugs on SNAP-25 and synaptophysin in the hippocampal trisynaptic pathway. Int J Neuropsychopharmacol 9:457–463
Barr CL, Feng Y, Wigg K, Bloom S, Roberts W, Malone M, Schachar R, Tannock R, Kennedy JL (2000) Identification of DNA variants in the SNAP-25 gene and linkage study of these polymorphisms and attention-deficit hyperactivity disorder. Mol Psychiatry 5:405–409
Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA (2002) Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry 159:1018–1028
Bleuler E (1950) Dementia Praecox for the Group of Schizophrenias. In: Zinkin J (ed) International University Press, New York
Brophy K, Hawi Z, Kirley A, Fitzgerald M, Gill M (2002) Synaptosomal-associated protein 25 (SNAP-25) and attention deficit hyperactivity disorder (ADHD): evidence of linkage and association in the Irish population. Mol Psychiatry 7:913–917
Burdick KE, Goldberg JF, Harrow M, Faull RN, Malhotra AK (2006) Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. J Nerv Ment Dis 194:255–260
Burdick KE, Goldberg TE, Funke B, Bates JA, Lencz T, Kucherlapati R, Malhotra AK (2007) DTNBP1 genotype influences cognitive decline in schizophrenia. Schizophr Res 89:169–172
Dudbridge F (2003) A survey of current software for linkage analysis. Hum Genomics 1:63–65
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98:6917–6922
Erlenmeyer-Kimling L, Cornblatt BA, Rock D, Roberts S, Bell M, West A (1993) The New York high-risk project: anhedonia, attentional deviance, and psychopathology. Schizophr Bull 19:141–153
Gold JM, Carpenter C, Randolph C, Goldberg TE, Weinberger DR (1997) Auditory working memory and Wisconsin card sorting test performance in schizophrenia. Arch Gen Psychiatry 54:159–165
Goldberg TE, Straub RE, Callicott JH, Hariri A, Mattay VS, Bigelow L, Coppola R, Egan MF, Weinberger DR (2006) The G72/G30 gene complex and cognitive abnormalities in schizophrenia. Neuropsychopharmacology 31:2022–2032
Gonzalez-Blanch C, varez-Jimenez M, Rodriguez-Sanchez JM, Perez-Iglesias R, Vazquez-Barquero JL, Crespo-Facorro B (2006) Cognitive functioning in the early course of first-episode schizophrenia spectrum disorders: timing and patterns. Eur Arch Psychiatry Clin Neurosci 256:364–371
Gosso MF, de Geus EJ, van Belzen MJ, Polderman TJ, Heutink P, Boomsma DI, Posthuma D (2006) The SNAP-25 gene is associated with cognitive ability: evidence from a family-based study in two independent Dutch cohorts. Mol Psychiatry 11:878–886
Green MF, Marshall BD Jr, Wirshing WC, Ames D, Marder SR, McGurk S, Kern RS, Mintz J (1997) Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am J Psychiatry 154:799–804
Gutbrod K, Cohen R, Mager B, Meier E (1989) Coding and recall of categorized material in aphasics. J Clin Exp Neuropsychol 11:821–841
Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, Lipska BK (2003) Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry 8:797–810
Harrison PJ, Weinberger DR (2005) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10:40–68
Hawkins KA (1999) Memory deficits in patients with schizophrenia: preliminary data from the Wechsler memory scale-third edition support earlier findings. J Psychiatry Neurosci 24:341–347
Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV (2001) Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry 58:24–32
Hess EJ, Collins KA, Copeland NG, Jenkins NA, Wilson MC (1994) Deletion map of the coloboma (Cm) locus on mouse chromosome 2. Genomics 21:257–261
Heyser CJ, Wilson MC, Gold LH (1995) Coloboma hyperactive mutant exhibits delayed neurobehavioral developmental milestones. Brain Res Dev Brain Res 89:264–269
Honer WG, Falkai P, Young C, Wang T, Xie J, Bonner J, Hu L, Boulianne GL, Luo Z, Trimble WS (1997) Cingulate cortex synaptic terminal proteins and neural cell adhesion molecule in schizophrenia. Neuroscience 78:99–110
Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Hoschl C (2006) Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs 20:389–409
Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JG, Griffin WS (1999) Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for “hypofrontality”. Mol Psychiatry 4:39–45
Keefe RS, Roitman SE, Harvey PD, Blum CS, DuPre RL, Prieto DM, Davidson M, Davis KL (1995) A pen-and-paper human analogue of a monkey prefrontal cortex activation task: spatial working memory in patients with schizophrenia. Schizophr Res 17:25–33
Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RR, Yurgelun-Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA (2004) Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry 161:985–995
Keefe RS, Sweeney JA, Gu H, Hamer RM, Perkins DO, McEvoy JP, Lieberman JA (2007) Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry 164:1061–1071
Kern RS, Green MF, Cornblatt BA, Owen JR, McQuade RD, Carson WH, Ali M, Marcus R (2006) The neurocognitive effects of aripiprazole: an open-label comparison with olanzapine. Psychopharmacology (Berl) 187:312–320
Kornhuber J, Schultz A, Wiltfang J, Meineke I, Gleiter CH, Zochling R, Boissl KW, Leblhuber F, Riederer P (1999) Persistence of haloperidol in human brain tissue. Am J Psychiatry 156:885–890
Kraepelin E (1919) Dementia Praecox and Paraphrenia. In: Barclay RM, Robertson GM (eds) Lifingstone, Edinborough
Kustanovich V, Merriman B, McGough J, McCracken JT, Smalley SL, Nelson SF (2003) Biased paternal transmission of SNAP-25 risk alleles in attention-deficit hyperactivity disorder. Mol Psychiatry 8:309–315
Lehrl S (1977) Mehrfachwahl-Wortschatz-Intelligenztest. Manual zum MWT-B. Manual zum MWT-B. Straube, Erlangen
Lewis R (2004) Should cognitive deficit be a diagnostic criterion for schizophrenia? J Psychiatry Neurosci 29:102–113
Meltzer HY (2004) Cognitive factors in schizophrenia: causes, impact, and treatment. CNS Spectr 9:15–24
Meltzer HY, McGurk SR (1999) The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull 25:233–255
Moritz S, Woodward TS, Krausz M, Naber D (2002) Relationship between neuroleptic dosage and subjective cognitive dysfunction in schizophrenic patients treated with either conventional or atypical neuroleptic medication. Int Clin Psychopharmacol 17:41–44
Morrens M, Wezenberg E, Verkes RJ, Hulstijn W, Ruigt GS, Sabbe BG (2007) Psychomotor and memory effects of haloperidol, olanzapine, and paroxetine in healthy subjects after short-term administration. J Clin Psychopharmacol 27:15–21
Mukaetova-Ladinska EB, Hurt J, Honer WG, Harrington CR, Wischik CM (2002) Loss of synaptic but not cytoskeletal proteins in the cerebellum of chronic schizophrenics. Neurosci Lett 317:161–165
Muller DJ, Klempan TA, De LV, Sicard T, Volavka J, Czobor P, Sheitman BB, Lindenmayer JP, Citrome L, McEvoy JP, Lieberman JA, Honer WG, Kennedy JL (2005) The SNAP-25 gene may be associated with clinical response and weight gain in antipsychotic treatment of schizophrenia. Neurosci Lett 379:81–89
Muller N, Schwarz M (2006) Schizophrenia as an inflammation-mediated dysbalance of glutamatergic neurotransmission. Neurotox Res 10:131–148
Nakahara T, Nakamura K, Tsutsumi T, Hashimoto K, Hondo H, Hisatomi S, Motomura K, Uchimura H (1998) Effect of chronic haloperidol treatment on synaptic protein mRNAs in the rat brain. Brain Res Mol Brain Res 61:238–242
Nuechterlein KH, Dawson ME, Green MF (1994) Information-processing abnormalities as neuropsychological vulnerability indicators for schizophrenia. Acta Psychiatr Scand Suppl 384:71–79
Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC (1989) The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol 109:3039–3052
Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste DV (1997) Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology 11:437–446
Peuskens J, Demily C, Thibaut F (2005) Treatment of cognitive dysfunction in schizophrenia. Clin Ther 27(Suppl A):25–37
Rapoport JL, Addington AM, Frangou S, Psych MR (2005) The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry 10:434–449
Reilly JL, Harris MS, Keshavan MS, Sweeney JA (2006) Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch Gen Psychiatry 63:1189–1197
Reitan RM (1958) Validity of the trailmaking test as an indication of organic brain damage. Percept Mot Skills 8:271–276
Rey A (1958) Ĺexamen clinique en psychologie. Presse Universitaire de France, Paris
Riedel M, Spellmann I, Strassnig M, Douhet A, Dehning S, Opgen-Rhein M, Valdevit R, Engel RR, Kleindienst N, Mueller N, Moeller HJ (2007) Effects of Risperidone and Quetiapine on cognition in patients with schizophrenia and predominantly negative symptoms. Eur Arch Psychiatry Clin Neurosci 257(6):360–370
Riedel M, Muller N, Spellmann I, Engel RR, Musil R, Valdevit R, Dehning S, Douhet A, Cerovecki A, Strassnig M, Moller HJ (2007) Efficacy of olanzapine versus quetiapine on cognitive dysfunctions in patients with an acute episode of schizophrenia. Eur Arch Psychiatry Clin Neurosci
Ruiz JC, Soler MJ, Fuentes I, Tomas P (2007) Intellectual functioning and memory deficits in schizophrenia. Compr Psychiatry 48:276–282
Rybakowski JK, Borkowska A, Skibinska M, Szczepankiewicz A, Kapelski P, Leszczynska-Rodziewicz A, Czerski PM, Hauser J (2006) Prefrontal cognition in schizophrenia and bipolar illness in relation to Val66Met polymorphism of the brain-derived neurotrophic factor gene. Psychiatry Clin Neurosci 60:70–76
Sawada K, Young CE, Barr AM, Longworth K, Takahashi S, Arango V, Mann JJ, Dwork AJ, Falkai P, Phillips AG, Honer WG (2002) Altered immunoreactivity of complexin protein in prefrontal cortex in severe mental illness. Mol Psychiatry 7:484–492
Snitz BE, Macdonald AW III, Carter CS (2006) Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull 32:179–194
Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature 362:318–324
Spreen O, Benton AL (1965) Comparative studies of some psychological tests for cerebral damage. J Nerv Ment Dis 140:323–333
Thoma P, Wiebel B, Daum I (2007) Response inhibition and cognitive flexibility in schizophrenia with and without comorbid substance use disorder. Schizophr Res 92:168–180
Thompson PM, Egbufoama S, Vawter MP (2003) SNAP-25 reduction in the hippocampus of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 27:411–417
Thompson PM, Kelley M, Yao J, Tsai G, van Kammen DP (2003) Elevated cerebrospinal fluid SNAP-25 in schizophrenia. Biol Psychiatry 53:1132–1137
Velligan DI, Prihoda TJ, Sui D, Ritch JL, Maples N, Miller AL (2003) The effectiveness of quetiapine versus conventional antipsychotics in improving cognitive and functional outcomes in standard treatment settings. J Clin Psychiatry 64:524–531
Wagner M, Quednow BB, Westheide J, Schlaepfer TE, Maier W, Kuhn KU (2005) Cognitive improvement in schizophrenic patients does not require a serotonergic mechanism: randomized controlled trial of olanzapine vs amisulpride. Neuropsychopharmacology 30:381–390
Watanabe A, Kato T (2004) Cerebrovascular response to cognitive tasks in patients with schizophrenia measured by near-infrared spectroscopy. Schizophr Bull 30:435–444
Wechsler D (1986) Wechsler Intelligence Scale for Childre-Revised (WISC-R)
Wiebel B, Happe A, Piekara FH (1995) Das neuropsychologische Diagnostikprogramm TESTBAT
Wong AH, Macciardi F, Klempan T, Kawczynski W, Barr CL, Lakatoo S, Wong M, Buckle C, Trakalo J, Boffa E, Oak J, Azevedo MH, Dourado A, Coelho I, Macedo A, Vicente A, Valente J, Ferreira CP, Pato MT, Pato CN, Kennedy JL, Van Tol HH (2003) Identification of candidate genes for psychosis in rat models, and possible association between schizophrenia and the 14-3-3eta gene. Mol Psychiatry 8:156–166
Zuffante P, Leonard CM, Kuldau JM, Bauer RM, Doty EG, Bilder RM (2001) Working memory deficits in schizophrenia are not necessarily specific or associated with MRI-based estimates of area 46 volumes. Psychiatry Res 108:187–209
Acknowledgments
We thank the following persons for their excellent assistance: Judith Nuernberger, Nadine Schaaf, Ricarda Zimmermann, Markus Opgen-Rhein, Katja Maino, Rebecca Schennach, Larissa de la Fontaine, Mitja Jandl, Stefanie Behrens, Sylvia de Jonge, Karin Neumeier.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spellmann, I., Müller, N., Musil, R. et al. Associations of SNAP-25 polymorphisms with cognitive dysfunctions in Caucasian patients with schizophrenia during a brief trail of treatment with atypical antipsychotics. Eur Arch Psychiatry Clin Neurosc 258, 335–344 (2008). https://doi.org/10.1007/s00406-007-0800-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-007-0800-9