Abstract
Introduction
There has been considerable discussion whether clinical trials accurately depict everyday practice. Restrictive inclusion/exclusion criteria, ethical considerations, differences in the severity of psychopathology between clinical and trial patients, or safety issues may bias results, which in turn may rather represent outcome for the “ideal” than for the “average” patient. Therefore, translation into psychiatric practice may be difficult.
Methods
A retrospective case–control study was performed. Schizophrenia inpatients at the LMU Department of Psychiatry, Munich, Germany, who had participated in clinical trials were compared to regular patients serving as controls. Probands and controls were matched by DSM–IV diagnosis, gender and age. The AMDP module, CGI and GAF were used to compare psychopathology. In addition, charts were reviewed for medication dosages, concurrent medical and neurological illness, and clinical history such as age of onset or family history.
Results
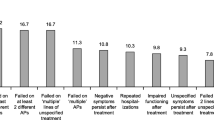
A total of 200 probands (100/100) were enrolled in the study. With respect to psychopathology, formally thought disordered or suicidal patients were significantly less likely to be study participants (n = 3) than controls (n = 22; p ≤ 0.05). Similarly, negative schizophrenia symptoms were significantly less often present in study participants (n = 17) than in controls (n = 38; p ≤ 0.05). Study participants were also medically and neurologically healthier than controls. (p = 0.05 respectively). No differences in overall illness severity as depicted by CGI and GAF were observed.
Conclusion
We found the patients included in our clinical trials representative of the patient encountered in routine clinical practice. Adherence to inclusion and exclusion criteria prevents inclusion of severely ill (e. g. suicidal) patients requiring a more intensive treatment setting. Illness severity was found to be similar in trial participants and controls, and indicates an overall comparably severe psychopathology. The more chronic, rather treatment refractory patients were also not reflected in the trial participant pool; this population may arguably not represent the average clinical patient either. A more careful administration of antipsychotic medication was found in trial participants and may effectively be considered “good clinical practice”.
Similar content being viewed by others
References
AMDP (1981) Das AMDP-System. Manual zur Dokumentation psychiatrischer Befunde. Berlin – Heidelberg – New York: Springer
American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.Washington, DC,American Psychiatric Association
Angst J, Scharfetter C, Stassen HH (1989) Classification of schizo-affective patients by multidimensional scaling and cluster analysis. Psychiatr Clin 16:254–264
Arnold LE, Hoagwood K, Jensen PS, Vitiello B (1997) Toward clinically relevant clinical trials. Psychopharmacol Bull 33(1):135–142
Benkert O, Hippius H (1996) Psychiatric Pharmacotherapy. 6th edition. Berlin – Heidelberg – New York – Tokyo, Springer, pp 226–277
Bobon D, Baumann U, Angst J, Helmchen H, Hippius H (eds) (1983) The AMDP-system in pharmacopsychiatry. Basel: Karger
Brown S,Birthwistle J,Roe L, Thompson C (1999) The unhealthy lifestyle of people with schizophrenia. Psychol Med 29(3):697–701
Endicott J, Spitzer RL, Fleiss JL, Cohen J (1976) The Global Assessment Scale – a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 33:766–771
Fisher WH,Barreira PJ,Geller JL,White AW,Lincoln AK,Sudders M (2001) Long-stay patients in state psychiatric hospitals at the end of the century. Psychiatr Serv 52(8):1051–1056
Frank E, Rush AJ, Blehar M, Essock S, Hargreaves W, Hogan M, et al. (2002) Skating to where the puck is going to be: a plan for clinical trials and translation research in mood disorders. Biol Psychiatry 52:631–654
Gebhardt R, Pietzcker A, Strauss A, Stoeckel M, Langer C, Freudenthal A (1983) Skalenbildung im AMDP-System. Arch Psychiatr Nervenkr 233:223–245
Goldman LS (1999) Medical illness in patients with schizophrenia. J Clin Psychiatry (Suppl 21):10–15
Guy GW, Ban TA (1982) The AMDP system. Manual for the assessment and documentation of psychopathology. Berlin – Heidelberg – New York: Springer
Guy W (1976) ECDEU Assessment manual for psychopharmacology. US Dept of Health and Human Services publication ADM 76(338), pp 524–535
Heimann H (1983) From Kraepelin to the system of the Association for Methodology and Documentation in Psychiatry. Acta Psychiatr Belg 83(2):147–154
Hellewell JS (1999) Treatment-resistant schizophrenia: reviewing the options and identifying the way forward. J Clin Psychiatry 60(Suppl 23):14–19
Hofer A, Hummer M, Huber R, Kurz M,Walch T, Fleischhacker WW (2000) Selection Bias in Clinical Trials With Antipsychotics. J Clin Psychopharmacol 20:699–702
Jahn T, Mussgay L (1989) Die stationäre Kontrolle möglicher Medikamenteneinflüsse in experimental-psychologischen Schizophreniestudien: Ein Vorschlag zur Berechnung von Chlorpromazin-Äquivalenten. Zeitsch Klein Psychologie 3:257–267
Jeste DV, Gladsjo JA, Lindamer LA, Lacro JP (1996) Medical comorbidity in schizophrenia. Schiz Bull 22(3):413–430
Leber PD,Davis CS (1998) Threats to the Validity of Clinical Trials Employing enrichment strategies for sample selection. Controlled Clinical Trials 19:178–187
Keith SJ (2001) Evaluating characteristics of patient selection and dropout rates. J Clin Psychiatry 62(Suppl 9):11–14
Nasrallah HA,Mulvihill T (2001) Iatrogenic disorders associated with conventional vs. atypical antipsychotics. Ann Clin Psychiatry 13(4):215–227
Perkins DO (2002) Predictors of non-compliance in patients with schizophrenia. J Clin Psychiatry 63(12):1121–1128
Renfordt E, Busch H, v Cranach M, Gulbinat W, Tegeler J (1983) Particular aspects of the interrater reliability of the AMDP psychopathology scale. In: Bobon D, Baumann U, Angst J, Helmchen H, Hippius H (eds) AMDP-system in pharmacopsychiatry. Modern problems of pharmacopsychiatry 20. Basel: Karger, pp 119–124
Robinson D, Woerner MG, Pollack S, Lerner G (1996) Subject selection Biases in Clinical Trials: Data From a Multicenter Schizophrenia Treatment Study. J Clin Psychopharmacol 16:170–176
Van Os J, Fahy T, Jones P, Harvey I, Toone B, Murray R (1997) Tardive Dyskinesia: who is at risk? Acta Psychiatr Scand 96(3):206–216
Wells KB (1999) Treatment Research at the Crossroads: The Scientific Interface of Clinical Trials and Effectiveness Research. Am J Psychiatry 156:5–10
Wirshing DA, Pierre JM, Erhart SM, Boyd JA (2003) Understanding the new and evolving profile of adverse drug effects in schizophrenia. Psychiatr Clin North Am 26(1):165–190
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riedel, M., Strassnig, M., Müller, N. et al. How representative of everyday clinical populations are schizophrenia patients enrolled in clinical trials?. Eur Arch Psychiatry Clin Neurosci 255, 143–148 (2005). https://doi.org/10.1007/s00406-004-0547-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-004-0547-5