Abstract
Currently available data regarding the blood levels of erythropoietin (EPO) in sleep apnea (SA) patients are contradictory. The aim of the present meta-analysis was to evaluate the EPO levels in SA patients via quantitative analysis. A systematic search of Pubmed, Embase, and Web of Science were performed. EPO levels in SA group and control group were extracted from each eligible study. Weight mean difference (WMD) or Standard mean difference (SMD) with 95% confidence interval (CI) was calculated by using fixed-effects or random effect model analysis according to the degree of heterogeneity between studies. A total of 9 studies involving 407 participants were enrolled. The results indicated that EPO levels in SA group were significantly higher than that in control group (SMD 0.61, 95% CI 0.11–1.11, p = 0.016). Significantly higher EPO levels were found in patients with body mass index <30 kg/m2, and cardiovascular complications in the subsequent subgroup analysis (both p < 0.05). High blood EPO levels were found in SA patients in the present meta-analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sleep apnea (SA), namely, is the absence of oronasal air flow during sleep. The pathophysiologic characteristics of SA are chronic intermittent hypoxia and sleep fragmentation [1]. SA is a highly prevalent disorder among adults [2]. SA is divided to obstructive sleep apnea (OSA) and central sleep apnea (CSA). The former is characterized by recurrent collapse of upper airway, whereas the latter is caused by the unstable ventilation drive [1, 3]. Abundant evidence confirmed that SA is firmly associated with increased risk of cardiovascular disease and mortality [4, 5]. Studies also suggested that SA might influence the levels of hematocrit [6] and blood viscosity [7]. The correlation between SA and polycythemia has also been reported [8].
Erythropoietin (EPO) is a glycoprotein hormone which is synthesized primarily by the kidney in adult. It is widely recognized that EPO may stimulate erythroid stem cells of the bone marrow to proliferate and differentiate. EPO has also been identified to play an important role in the mechanism of cardiovascular diseases. Robust evidence shows that EPO appears to be released in response to hypoxia [9].
Whether the EPO levels in SA patients being changed or not remains controversial. Some studies demonstrated that EPO concentrations were no different between SA patients and normal subjects [10, 11]. In other studies, however, authors claimed that EPO levels were significantly increased in SA subjects [12, 13]. Furthermore, it has been suggested that continuous positive airway pressure (CPAP) treatment might normalize the diurnal EPO levels in SA patients [14].
The primary aim of the present meta-analysis was to evaluate the EPO levels in SA patients via quantitatively analysis the present available literature.
Materials and methods
The present meta-analysis was conducted following the guideline of the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) [15].
Search strategy
The electronic databases, namely PubMed, Embase, and Web of Science, were searched by using the following terms: sleep apnea OR sleep-disorder breathing, and erythropoietin from inception to May, 4, 2016. No language restrictions were applied. References from included studies were also perused.
Literature selection criteria
Two reviewers independently selected the available studies. Studies which met the following criteria were enrolled into the present meta-analysis: (1) participants included in the study were adults; (2) diagnosis of SA was according to polysomnography (PSG); (3) EPO levels were reported both in SA group and control group. Editorials, reviews, case reports, congress articles, and animal studies were excluded. An email to the corresponding author was written if the essential data of study was ambiguous or not presented. After two no response attempt, the study was also ruled out. The consensus was obtained through a meeting with all authors if any discrepancy were presented between the two reviewers.
Literature quality and data extraction
Evidence level was defined in accordance with Oxford Centre for Evidence-based Medicine (CEBM)-Levels of Evidence [16, 17]. The data of the included articles were extracted by two reviewers independently. The following items were listed: first author, publication year, country, sample size, and the clinical characteristics of the participants, such as age, male percentage, body mass index (BMI), complications, PSG parameters and EPO levels in each group.
Statistical analysis
Stata version 12.0 and Review Manager 5.2 were applied for statistical analysis. The I 2 was obtained to express the heterogeneity between studies. If I 2 > 50%, indicating that moderately or highly heterogeneous existed, a random effects model was conducted to estimate the effect size (Standard mean difference, SMD; 95% confidence interval, 95% CI); if I 2 ≤ 50%, fixed-effects model was used to obtain the weighted mean difference (WMD, 95% confidence interval, 95% CI) [18]. Furthermore, subgroup analysis was performed to assess the influence of gender, BMI, AHI, cardiovascular disease, and time of exsanguinate blood on EPO levels. Since these variables of other type of SA [19, 20] and ODI value [21, 22], were only reported in two included studies, subgroup analysis were not performed stratified by these two variables. Sensitivity analysis was also conducted to evaluate the influence of each study on overall effect size. Potential publication bias was showed with funnel plot, and tested by Begg’s test and Egger’s test [23]. Statistical significance was confirmed if p value <0.05.
Results
Literature search
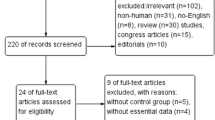
A total of 405 studies were identified by electronic search for initial scrutiny. After removing duplicated records and reviewing the titles and abstracts, 24 studies were considered worthy of further full-text scrutiny. Of the 24 studies, 15 studies were subsequently excluded (see detail in Fig. 1). Finally, 9 studies [10–13, 19–21, 24, 25] involving 407 participants were included into the present meta-analysis.
Characteristics of included studies
Tables 1 and 2 outline the characteristics of the subjects in each study. The evidence levels were 2b (both evidence levels and recommendation grade were moderate according to CEBM) in all studies. Sample size in each study was relatively small (less than 100). The predominant type of SA was obstructive sleep apnea (OSA) in most of the included studies. Except for one study, all SA patients in the included studies suffered severe SA (the apnea hypopnea index was more than 30 events/h). The exact EPO data in one study was obtained via contacting the corresponding author [25].
Pool analysis of the difference in EPO between SA group and control group
As the I 2 (80.2%) was significantly high, obviously heterogeneous existed between studies, thus random effect model was conducted to explore the difference in EPO between groups. Figure 2 illustrates that EPO levels in the SA group were significantly higher than that in control group (SMD 0.61, 95% CI 0.11–1.11, p = 0.016).
Subgroup analysis stratified by various clinical parameters
Table 3 outlines the subgroup analysis stratified by different parameters. Results showed that there were significantly higher EPO levels in patients with BMI <30 kg/m2, and cardiovascular complications. EPO levels were increased in patients with SA regardless of AHI levels. Gender, and exsanguinate time had no influence on EPO in the further subgroup analysis.
Sensitivity analysis
Sensitivity analysis showed that individual study had no influence on the overall effect size (Fig. 3).
Publication bias
Figure 4 shows that publication bias seem to exist, however, both Begg and Egger tests proved that no publication bias existed in the present study (p = 0.293 and 0.466, respectively).
Discussion
The present meta-analysis with 9 studies involving 407 participants demonstrated that, compared with normal subjects, EPO levels were increased in patients with SA, especially in those with low BMI, and cardiovascular complications.
EPO, a glycoprotein hormone with 30.4 kDa relative molecular mass, is composed of 165 amino acids. It is mainly produced by the kidney and liver in adult [26]. EPO can bind to erythroid progenitor cell surface receptor, then leading to the activation of several signal pathways, such as the Ras/mitogen-activated kinase pathway, etc [27]. After stimulation of certain genes expression, erythropoietic progenitor cells can proliferate and differentiate to mature red blood cells. The vital function of EPO is of regulation the blood oxygen levels via adjusting the circulating erythrocytes number [26]. Diurnal variation of serum EPO levels can be detected in normal subjects: the nadir occurred at daytime, the peak concentration happened early in the morning, but this phenomenon could not be observed in chronic obstructive pulmonary disease [28, 29].
Serum levels of EPO can be influenced by various factors. The most important one is hypoxia. Evidence showed that elevated serum EPO levels are an adaptive response of human body to hypoxia. A previous study indicated that COPD patients, characterized by sustained hypoxia, had elevated serum EPO levels [30]. The pathophysiological mechanism of intermittent hypoxia is similar to ischemia/reperfusion injury. Several studies indicated that pre-exposure to intermittent hypoxia can protect the myocardial tissue against ischemia/reperfusion injury [31, 32]. Similarly to sustained hypoxia, intermittent hypoxia has also been found to play a magnificent role in the regulation of EPO levels. An experimental study illustrated that EPO was increased significantly when rats were exposed to intermittent hypoxia for 1–3 weeks [33]. The vital pathophysiological characteristic of SA is intermittent hypoxia. However, whether the EPO levels are increased or not in SA patients remained controversial. In addition, some interventional studies on the effect of CPAP treatment on EPO levels have shown contradictory results. Cahan et al. [14] showed that CPAP treatment might attenuate diurnal EPO levels in SA patients. A study by Ryan and coworkers [25] demonstrated that the EPO levels in OSA patients had no alternation after 6 weeks of CPAP treatment. Multicenter, randomized-control interventional study is needed to clarify the definitive effect of CPAP on EPO levels.
The subgroup analysis of the present study indicated that SA patients with cardiovascular disease had significantly higher EPO levels. We speculated that elevated EPO levels in patients with SA and cardiovascular disease were an adaptive response against intermittent hypoxia [31]. Previous studies claimed that EPO levels had diurnal variation [12, 24]. The present meta-analysis was inconsistent with those studies, in that we could not observe the circadian fluctuation of EPO in SA patients. We also failed to explain the phenomenon that when compared to patients with high BMI, EPO was increased in patients with low BMI. The low numbers of included studies and small sample size might partly contribute to those phenomena. Further investigation is required to clarify those aforementioned phenomena.
Several limitations of the present meta-analysis should be emphasized. First, the most significant limitation was the severe heterogeneity between included studies, showing a high variation of the results among each study. Second, the individual study is relatively low-level evidence. Third, although a highly sensitive search strategy for the potentially eligible studies was applied, some studies may still be overlooked. Fourth, the relative small sample size of each included study might restrict the generalizability of the results. Fifth, except two included studies [19, 20] (subjects in one study was CSA, the other one was sleep-disorder breathing), subjects in the most remain studies were OSA, it was difficult for us to evaluate the influence of SA type on serum EPO levels. Finally, although statistical significance was not observed, it was still hard to rule out the publication bias.
In conclusion, the present meta-analysis confirmed that elevated EPO levels were found in SA patients. We speculated that increased EPO might be associated with increased risk of subsequent cardiovascular diseases in SA patients.
References
Jordan AS, McSharry DG, Malhotra A (2014) Adult obstructive sleep apnoea. The Lancet 383(9918):736–747. doi:10.1016/S0140-6736(13)60734-5
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328(17):1230–1235. doi:10.1056/NEJM199304293281704
Bradley TD, Floras JS (2003) Sleep apnea and heart failure: Part II: central sleep apnea. Circulation 107(13):1822–1826. doi:10.1161/01.CIR.0000061758.05044.64
Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G (2014) Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med 11(2):e1001599. doi:10.1371/journal.pmed.1001599
Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, Soler-Cataluna JJ, Almeida-Gonzalez C, De la Cruz Moron I, Duran-Cantolla J, Montserrat JM (2012) Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med 186 (9):909–916. doi:10.1164/rccm.201203-0448OC
Choi JB, Loredo JS, Norman D, Mills PJ, Ancoli-Israel S, Ziegler MG, Dimsdale JE (2006) Does obstructive sleep apnea increase hematocrit? Sleep Breath 10(3):155–160
Bernath I, McNamara P, Szternak N, Szakacs Z, Koves P, Terray-Horvath A, Vida Z (2009) Hyperviscosity as a possible cause of positive acoustic evoked potential findings in patients with sleep apnea: a dual electrophysiological and hemorheological study. Sleep Med 10(3):361–367. doi:10.1016/j.sleep.2008.03.012
Pathak R, Giri S, Karmacharya P, Aryal MR (2015) Obstructive sleep apnea syndrome and secondary polycythemia: analysis of the nationwide inpatient sample. Sleep Med 16(1):205–206. doi:10.1016/j.sleep.2014.09.012
Barbosa C, Romao L (2014) Translation of the human erythropoietin transcript is regulated by an upstream open reading frame in response to hypoxia. RNA 20(5):594–608. doi:10.1261/rna.040915.113
Pokala P, Llanera M, Sherwood J, Scharf S, Steinberg H (1995) Erythropoietin response in subjects with obstructive sleep-apnea. Am J Respir Crit Care Med 151(6):1862–1865
Ciftci TU, Kokturk O, Demirtas S, Gulbahar O, Bukan N (2011) Consequences of hypoxia-reoxygenation phenomena in patients with obstructive sleep apnea syndrome. Ann Saudi Med 31(1):14–18
Cahan C, Decker MJ, Arnold JL, Washington LH, Veldhuis JD, Goldwasser E, Strohl KP (1992) Diurnal variations in serum erythropoietin levels in healthy subjects and sleep apnea patients. J Appl Physiol (1985) 72 (6):2112–2117
Imagawa S, Yamaguchi Y, Higuchi M, Neichi T, Hasegawa Y, Mukai HY, Suzuki N, Yamamoto M, Nagasawa T (2001) Levels of vascular endothelial growth factor are elevated in patients with obstructive sleep apnea-hypopnea syndrome. Blood 98(4):1255–1257
Cahan C, Decker MJ, Arnold JL, Goldwasser E, Strohl KP (1995) Erythropoietin levels with treatment of obstructive sleep apnea. J Appl Physiol (1985) 79 (4):1278–1285
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012. doi:10.1016/j.jclinepi.2009.06.005
OCEBM levels of Evidence Working Group (2009) The Oxford 2009 levels of evidence. Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/indexaspx?o=5653
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schunemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S, Group GW (2004) Grading quality of evidence and strength of recommendations. BMJ 328(7454):1490. doi:10.1136/bmj.328.7454.1490
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
Calvin AD, Somers VK, Steensma DP, Rio Perez JA, Van Der Walt C, Fitz-Gibbon JM, Scott CG, Olson LJ (2010) Advanced heart failure and nocturnal hypoxaemia due to central sleep apnoea are associated with increased serum erythropoietin. Eur J Heart Fail 12(4):354–359
Kukwa W, Glowczynska R, Filipiak KJ, Kukwa A, Opolski G, Budaj-Fidecka A, Grabowski M, Galazka A, Krzeski A, Kuzminska M, Czarnecka AM (2013) Serum EPO and VEGF levels in patients with sleep-disordered breathing and acute myocardial infarction. Sleep Breath 17(3):1063–1069
McKeon JL, Saunders NA, Murree-Allen K, Olson LG, Gyulay S, Dickeson J, Houghton A, Wlodarczyk J, Hensley MJ (1990) Urinary uric acid: Creatine ratio, serum erythropoietin, and blood 2,3-diphosphoglycerate in patients with obstructive sleep apnea. Am Rev Respir Dis 142(1):8–13
Ryan S, Taylor CT, McNicholas WT (2005) Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112(17):2660–2667
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Wang YZ, Zhang FS (2004) Circadian rhythm of serum erythropoietin in obstructive sleep apnea/hypoventilation syndrome. Zhonghua Yi Xue Za Zhi 84(16):1379–1380
Ryan S, Taylor CT, McNicholas WT (2005) Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112(17):2660–2667. doi:10.1161/CIRCULATIONAHA.105.556746
Krantz SB (1991) Erythropoietin. Blood 77(3):419–434
Jelkmann W (2013) Physiology and pharmacology of erythropoietin. Transfus Med Hemother 40(5):302–309. doi:10.1159/000356193
Klausen T, Poulsen TD, Fogh-Andersen N, Richalet JP, Nielsen OJ, Olsen NV (1996) Diurnal variations of serum erythropoietin at sea level and altitude. Eur J Appl Physiol Occup Physiol 72(4):297–302
Casale R, Pasqualetti P (1997) Diurnal rhythm of serum erythropoietin circulating levels in chronic obstructive pulmonary disease. Panminerva Med 39(3):183–185
Markoulaki D, Kostikas K, Papatheodorou G, Koutsokera A, Alchanatis M, Bakakos P, Gourgoulianis KI, Roussos C, Koulouris NG, Loukides S (2011) Hemoglobin, erythropoietin and systemic inflammation in exacerbations of chronic obstructive pulmonary disease. Eur J Intern Med 22(1):103–107. doi:10.1016/j.ejim.2010.07.010
Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL (2003) Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108(1):79–85. doi:10.1161/01.CIR.0000078635.89229.8A
Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN (2003) Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res 13(5):385–391. doi:10.1038/sj.cr.7290184
Ishii M, Iwamoto T, Nagai A, Sasao G, Iwasaki M, Kuwahira I (2010) Polycythemia and changes in erythropoietin concentration in rats exposed to intermittent hypoxia. Adv Exp Med Biol 662:121–126. doi:10.1007/978-1-4419-1241-1_17
Acknowledgements
This work was supported by Grant 2013-2-88 for Youth Research Fund from Fujian Provincial Health Bureau and Grant 3502Z20154019 for Fund from Xiamen Science and Technology Bureau.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no conflict of interest.
Ethical approval
This study was a meta-analysis without human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhang, XB., Zeng, YM., Zeng, HQ. et al. Erythropoietin levels in patients with sleep apnea: a meta-analysis. Eur Arch Otorhinolaryngol 274, 2505–2512 (2017). https://doi.org/10.1007/s00405-017-4483-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-017-4483-1