Abstract
Smoking is the main causative factor for development of head and neck and lung cancer. In addition, other malignancies such as bladder, stomach, colorectal, kidney and pancreatic cancer have a causative relation with smoking. Continued smoking after having been diagnosed with cancer has many negative consequences: effectiveness of radiotherapy is diminished, survival time is shortened and risks of recurrence, second primary malignancies and treatment complications are increased. In view of the significant health consequences of continued smoking, therefore, additional support for patients to stop smoking seems a logical extension of the present treatment protocols for smoking-related cancers. For prospectively examining the effect of nursing-delivered smoking cessation programme for patients with head and neck or lung cancer, 145 patients with head and neck or lung cancer enrolled into this programme over a 2-year period. Information on smoking behaviour, using a structured, programme specific questionnaire, was collected at baseline, and after 6 and 12 months. At 6 months, 58 patients (40%) had stopped smoking and at 12 months, 48 patients (33%) still had refrained from smoking. There were no differences in smoking cessation results between patients with head and neck and lung cancer. The only significant factor predicting success was whether the patient had made earlier attempts to quit smoking. A nurse-managed smoking cessation programme for patients with head and neck or lung cancer shows favourable long-term success rates. It seems logical, therefore, to integrate such a programme in treatment protocols for smoking-related cancers.
Similar content being viewed by others
Introduction
Tobacco use in the form of cigarette, pipe or cigar smoking is associated with 5 million deaths per year worldwide. The estimation for 2025 is that this number will increase to 10 million deaths annually [1]. Furthermore, there appears to be a synergistic effect between smoking and alcohol intake. The relative risk of developing, for example, supraglottic laryngeal cancer is increased by 50% from what would be predicted by the simple additive effect of tobacco and alcohol abuse combined [2].
Smoking is the main causative factor for the development of head and neck and lung cancer. In addition, other malignancies, such as bladder, stomach, colorectal, kidney and pancreas cancer, have a causative relation with smoking [1, 3–5]. In the Netherlands, although a decline has been observed over the last decades, almost 30% of the population is still smoking.
To continue smoking after having been diagnosed with cancer has many negative consequences: the effectiveness of radiotherapy is diminished, survival time is shortened, and the risks of recurrence, second primary malignancies and treatment complications are increased. In a recent study from the Netherlands comprising 2012 patients, the effects of continued smoking on recurrence rate, secondary cancers, and on mortality was clearly shown (see Table 1) and the authors rightfully emphasise the importance of substance abuse cessation [6]. In a case–control study in 202 patients, Chen et al. [7] confirmed that tobacco smoking during radiation therapy for head-and-neck cancer was associated with unfavourable outcomes. Obviously, these complications and side effects also have a negative impact on the patient’s quality of life. Nevertheless, many patients, who were smoking prior to their illness, continue after diagnosis and treatment [8].
Although clinicians often warn about these smoking-related consequences and already at the time of diagnosis recommend their cancer patients to quit smoking, reports indicate that still 35–72% of patients continue smoking during and after treatment [9, 10]. Positively interpreted, these data indicate that fortunately, many smokers are able to stop without help and physicians and other health-care professionals apparently to some extent still have a positive influence on smoking cessation. This approach has reported threefold to fivefold increased cessation rates [11–13]. Furthermore, research has shown that patients suffering from serious disease may be more open for smoking cessation advice than smokers without serious health problems [14]. This means that patients receiving the diagnosis “cancer” probably are more receptive for such advise by their physician, and if that does not work, for an additional counselling programme. Better than in an initial patient-physician contact, such a counselling programme more comprehensively can and should be targeted towards dealing with the physical addiction to nicotine, the psychological reliance on the effects of nicotine, and the behavioural aspects of tobacco use [11].
In view of these significant health consequences of continued smoking, we considered that additional support for patients to stop smoking should become an integral part of the treatment protocol for those cancers that are clearly smoking related. For this reason, a ‘stop smoking clinic’ was initiated in the Netherlands Cancer Institute. In this paper, we assess whether such a clinic can contribute to smoking cessation in the patient population, who did not succeed in stopping solely on the basis of counselling by the health-care providers before the onset of their oncologic treatment. We will present the outcomes of this initiative with emphasis on the 12-month follow-up results and provide some data on the costs of the programme.
Patients and methods
Patients
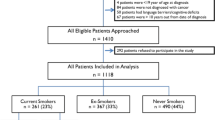
The project started as a so-called ‘Care-renewal project’ endorsed and funded by the health-care authorities and the study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. From November 2003 to December 2005, 185 patients visiting the hospital were referred to the ‘stop smoking clinic’. There were 16 patients who were excluded from the programme as they did not have cancer, and 24 patients, after having been informed about the programme, decided they did not (yet) want to participate, resulting in 145 patients for further enrolment in the programme, 78 (54%) males and 67 (46%) females. Of these patients, the majority had head-and-neck cancer (N = 96, 66%), followed by lung cancer (N = 34, 24%) and various other cancers (N = 15, 10%; breast cancer, sarcoma, or bladder cancer). The mean age patients started to smoke was 15 years, the mean consumption was 20 cigarettes per day and the mean tobacco exposure was 45 pack/years. All data were collected prospectively. Further detailed characteristics of the patients enrolled in the cessation programme are shown in Table 2. Patients were able to start with the programme at any time of their treatment, resulting in 45 patients (31%) starting the programme pre-treatment, (29) 20% during, and 71 (49%) post-treatment.
Counselling methods
The intervention programme applied is founded on the self-efficacy theory of Bandura, which is based on the patient’s self-efficacy (confidence in succeeding) and the changing model of Prochaska and DiClemente [15, 16]. We followed the seven steps of the Minimal Intervention Program of Stivoro (government organisation for stopping smoking in the Netherlands; http:\\www.stivoro.nl).
This theory-based nurse intervention programme consists of seven steps engaging the patients to stop smoking [17].
-
1.
Physician’s advice about the necessity to stop smoking.
-
2.
Data collection regarding smoking status by means of a structured questionnaire.
-
3.
Motivational interviewing of the patient to stop smoking.
-
4.
Inventory of barriers in the stop smoking period.
-
5.
Information about smoking cessation.
-
6.
Fixing a stop smoking date.
-
7.
Arrangement of follow-up and support.
The programme is most intensive in the first month, and lasts in total 1 year, in order to support the patient through several (annual) risk situations such as birthdays, stress situations and holidays. Detailed information (timetable) about the programme is shown in Table 3.
Since, in combination with alternative nicotine products (ANP), counselling appears to be an effective strategy to stop smoking, different products (nicotine lozenges, buproprion and combinations of products) were offered as well [17].
Cost assessment
The average counselling time needed for the one-year programme was assessed on the basis of the schedule applied and the costs of the counselling time by the specialists-nurse were calculated using the report of Hakkaart-van Roijen et al.; the costs for the nicotine replacements were derived from the Pharmacotherapeutic Compass (http://www.fk.cvz.nl/, nicotine replacements) [18, 19].
Statistical analyses
Data were entered into an SPSS database (version 15.0); the analysis is mainly descriptive. Differences are measured with the Chi square or the Fisher exact test, while differences between continuous variables have been measured by the Wilcoxon test (effect and results) or the Kruskal–Wallis test (months). Differences between the patient groups are measured by the Mantel–Heanszel test. A p value <0.05 was considered statistically significant.
Results
At 6 months, 58 patients (40%) had stopped smoking, 79 patients (54%) were still smoking, and 8 patients (6%) were lost to follow-up. After 12 months, 48 patients (33%) still had refrained from smoking, 60 patients (41%) had continued or were smoking again and 37 patients (26%) were ‘lost to follow-up’. Of these 37 patients, 5 died of disease, 13 had a recurrence, and 19 patients did not return for their one-year appointment (see Table 4). Of the 98 head-and-neck cancer patients, 30 (31%) and of the 32 lung cancer patients, 10 patients (31%) stopped smoking. Of the 60 patients, who did not quit smoking, 24 patients (22%) reported that they had reduced their number of cigarettes per day with 50% or more. No statistically significant effect with regards to the initial enrolment into the programme, i.e. pre-, during, or post-treatment, could be found: after 12 months, the number of patients in each of these three groups, who actually had stopped, was 13, 17 and 18 patients, respectively (p = 0.236). There was also no correlation of alcohol intake on the cessation rate (p = 0.588). Previously having tried to quit smoking, however, appeared to have a statistically significant positive influence. Patients, who had made earlier attempts to stop before entering into this programme, showed better smoking cessation results (n = 33, 69%) than patients, who never had tried to stop (n = 14, 29%, p < 0.05; data of one patient missing). There were no other statistical significant predictors for success, such as the motivation status of the patient at the start of the programme (assessed with a single question in the structured questionnaire, combining the answers a little to very motivated). In addition, no statistically significant association between the use of nicotine replacement products and smoking cessation could be established. During the 12-month programme, 91 of the 108 patients (84%) used some kind of nicotine replacement; the 12-month follow-up data are available (see Table 5). Furthermore, the data did not show an association between alcohol use and smoking cessation. Finally, neither the behaviour of the partner of the patient with respect to his/her own smoking (stopping or continuing), nor the extent of support by family and/or friends did appear to significantly affect the results.
This smoking cessation programme continued following our initial 2-year study period, and in Table 6 an overview is given of the number of since then included patients and their 12-month results. Although not further statistically analysed, it can be seen that except for 2006, when there was a dip in patients, staffing and experience with the programme, results are actually fairly constant, with 36% 1-year success in 2008.
Table 7 shows the mean time consumption and the personal costs for the patients in this programme. The total counselling time per patient varied between 130 and 200 min over the 1-year intervention programme, with a mean of 183 min per patient, or €255.74. Stated otherwise, one specialist-nurse in a 1-day per week appointment was able to manage the total patient cohort. The personal costs for the patients with regards to the used nicotine replacement patches, lozenges, and/or drugs varied between €22.46 for the use of one bottle of nicotine lozenges (which was the minimum) to €264.09 for the full combination of patches and drugs.
Discussion
The results of the ‘stop smoking clinic’, with smoking cessation of 40% of the patients at the 6-month interval and 33% at the 12-month interval are quite encouraging. In general, in the Netherlands the stop smoking results at 1 year are 29% for all adults [1]. While Simon et al. [21] had similar results (33% after 1 year) with intensive counselling and the use of free transdermal nicotine replacement products the smoking cessation rates (of head-and-neck cancer patients) of Gritz et al. [20] were exceptionally much higher, i.e. 70.2% after 1 year. These latter results most likely have been influenced by the inclusion of patients, who were already (less than 1 year) ex-smokers, and by the fact that 20 patients of the 114 in that study underwent a total laryngectomy, which makes smoking difficult or impossible [22]. In our patient cohort, neither there were total laryngectomy patients, nor did we include patients spontaneously quitting after their first visit to the clinic and the regular counselling by the physician. This means that there was a more ‘negative’ patient selection than in the study of Gritz [20].
An additional positive finding is that, although the intention of the stop smoking clinic is to completely stop and not to only reduce smoking, 24 out of the 60 patients (40% of this ‘failure’ group), who did not quit smoking, reported that they reduced their number of cigarettes per day with 50% or more. This still can be considered a worthwhile effect of the programme, since e.g. in a study of Godtfredsen et al. [23] it was demonstrated that reducing tobacco consumption from 20 to less than 10 cigarettes per day was associated with a 27% reduction in lung cancer risk compared with unchanged heavy smoking.
In our study, we did not find different smoking cessation results between head-and-neck cancer patients and lung cancer patients (both 31%), while Schnoll et al. [8] found a clear association between the cancer site and the smoking status: 21% of head-and-neck cancer patients versus 78% of lung cancer patients quit smoking in their study. An explanation for this difference might be that the lung cancer patients in our study mostly had disseminated disease and received palliative treatment. Most likely, this has reduced their motivation to quit smoking. Like Ostroff et al. [9], we did not find a correlation between the number of ‘smoking-years’, nor the daily consumption of tobacco and smoking cessation results.
Although our data did not show a statistically significant relation between the timing of the enrolment into the programme (pre-, during or post-treatment) and the actual smoking cessation rate, we still agree with Gritz et al. [20] that patients should start such a stop smoking programme as early as possible, i.e. preferably at the time of diagnosis. These authors stated that “the diagnosis and treatment period of head-and-neck cancer offer an opportune time for intervention, a “teachable moment”, when presumably there is a high motivation for cure and prevention of further disease”. Furthermore, Wewers et al. [24] reported that the diagnostic testing period for lung cancer is a good moment for promoting smoking abstinence. In addition, Rigotti et al. [25] concluded that smoking cessation interventions conducted with hospitalised patients were effective only when the outpatient follow-up period lasted for at least 1 month. Moreover, the study of Chen et al. [7] underlines that tobacco smoking during radiation therapy for head-and-neck cancer was associated with unfavourable outcomes, which is an additional reason to confront the patient as early as possible with smoking cessation. In contrast, patients who have completed their treatment might be tempted to minimise the seriousness of their diagnosis and, thus, might be less committed to quitting [8].
In our study patients, who had one or more earlier attempts to quit, have significantly better smoking cessation results. This is in agreement with Chan et al. [26], who in a multivariable modelling procedure showed that the number of attempts to quit smoking was significantly and independently related to smoking cessation. In addition, as stated by the US Public Health Service Clinical Practice Guideline update of 2008 [17]: “Tobacco dependence is a chronic disease that often requires repeated intervention and multiple attempts to quit”.
Prospective studies often show that motivation to quit smoking is a consistent predictor of smoking cessation and treatment studies show that initial ‘quit-motivation’ predicts success in a smoking intervention [23, 27]. However, in our study the motivation status of the patient at the start of the programme, assessed with a single item question in the Stivoro structured questionnaire (a little to very motivated) did not appear to be a significant factor in the stop smoking results. Probably, this single item question does not reflect the real motivation of the patient and only results in a socially acceptable answer.
Of the 12-month follow-up cohort, the vast majority of patients had used some kind of nicotine replacement, and that probably explains why no statistically significant association between the use of nicotine replacement products and quitting smoking habits could be established. This is in contrast to other studies, e.g. according to Hatsukami et al. [11] the combination of nicotine patches and the use of ad libitum nicotine lead to better efficacy than single products. In addition, the US Public Health Service Clinical Practice Guideline states that the combination of counselling and medication is more effective than either alone [17]. Presently, more anti-smoking drugs are available than at the start of the stop smoking clinic, and even nicotine vaccination (http:\\www.hag.unimaas.nl/nicotinevaccinstudie/UK/Home.htm) presently is trialled to support smoking cessation. Possibly these will further improve the results in stop smoking studies in the future.
Interestingly, our data did neither show an association between alcohol use and smoking cessation, nor with the patient’s partner smoking behaviour (continue or also trying to quit), or the extent of support by family and/or friends. This is in contrast with Schnoll et al. [8], who stated that having a family member at home who smokes increased the likelihood that patients will continue to smoke. They recommend recruiting the patient’s relatives, who smoke into the cessation programme. In our programme we also gave the relatives/partner advice how to help the patient with his/her attempts to stop smoking, and if the relatives/partner also wanted to quit smoking, they could follow the programme together with the patient. Maybe that is what they did and why we consequently did not see any influence of the relatives/partner smoking behaviour and the smoking cessation results of the patient. Finally, like Ostroff et al. [9], we neither found a relation between the number of pack/years nor with the daily consumption of tobacco and the smoking cessation results.
A limitation in this study is the subjectivity of the data regarding smoking status, which is based solely on patients’ self-report (questionnaires). Biochemical verification of self-reported smoking status via cotinine assays would strengthen the study findings. However, many quality of life studies report that smoking and alcohol habits of patients can be assessed correctly by means of questionnaires, but when it comes to the number of cigarettes or the amount of alcohol units per day you may assume that those self-reports are probably not reliable [9].
Another limitation of this study is that the study design and the data collected did not allow for a meaningful cost-effectiveness analysis. However, we think that quite likely the average counselling costs of €255,74 per patient outweigh the known disadvantages of continued smoking. Moreover, almost all cessation interventions targeted at smokers in the general population appear to be cost-effective [28]. Hoogendoorn et al. [29] e.g. reported that pharmacotherapy in combination with intensive counselling was cost saving compared with intensive counselling alone, and that the latter in turn was more effective than minimal intervention. These findings have resulted in the recent decision as of 2011 in The Netherlands to fully reimburse smoking cessation treatment programmes under the compulsory basic health insurance plans. This makes integration of such programmes in treatment plans obviously much easier.
In future, this stop smoking programme will be even more individualised: counselling moments related to highly and less dependent smokers, more focus on patients who never tried to quit before. Furthermore, since 2007 not only patients with head-and-neck cancer and lung cancer are included in the programme, but also patients with other types of cancer and e.g. patients requiring free flap surgery.
Conclusion
This nurse-managed smoking cessation programme for patients with head and neck or lung cancer, who were not able to stop despite specific counselling of their health-care providers before the onset of treatment, shows favourable results. Despite this negative selection, the long-term cessation rates seem even somewhat better than those achieved with comparable programmes for non-cancer patients in the Netherlands. The only significant factor for success appeared to be whether patients had made earlier attempts to stop smoking before entering in this programme. It seems logical, therefore, to integrate such a programme in treatment protocols for smoking-related cancers.
References
Bonneux L, Birnie E (2001) The discount rate in the economic evaluation of prevention: a thought experiment. J Epidemiol Community Health 55(2):123–125
Maier H, Weidauer H (1995) Alcohol drinking and tobacco smoking are the chief risk factors for ENT tumors. Increased incidence of mouth cavity, pharyngeal and laryngeal carcinomas. Fortschr Med 113(11):157–160
Brenner H, Rothenbacher D, Arndt V (2009) Epidemiology of stomach cancer. Methods Mol Biol 472:467–477. doi:10.1007/978-1-60327-492-0_23
Anderson B, Naish W (2008) Bladder cancer and smoking. Part 1: addressing the associated risk factors. Br J Nurs 17(18):1182–1186
Liang PS, Chen TY, Giovannucci E (2009) Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 124(10):2406–2415. doi:10.1002/ijc.24191
Farshadpour F, Kranenborg H, Calkoen EV, Hordijk GJ, Koole R, Slootweg PJ, Terhaard CH (2010) Survival analysis of head and neck squamous cell carcinoma: influence of smoking and drinking. Head Neck. doi:10.1002/hed.21549
Chen AM, Chen LM, Vaughan A, Sreeraman R, Farwell DG, Luu Q, Lau DH, Stuart K, Purdy JA, Vijayakumar S (2011) Tobacco smoking during radiation therapy for head-and-neck cancer is associated with unfavorable outcome. Int J Radiat Oncol Biol Phys 79(2):414–419. doi:10.1016/j.ijrobp.2009.10.050
Schnoll RA, Malstrom M, James C, Rothman RL, Miller SM, Ridge JA, Movsas B, Unger M, Langer C, Goldberg M (2002) Correlates of tobacco use among smokers and recent quitters diagnosed with cancer. Patient Educ Couns 46(2):137–145
Ostroff JS, Jacobsen PB, Moadel AB, Spiro RH, Shah JP, Strong EW, Kraus DH, Schantz SP (1995) Prevalence and predictors of continued tobacco use after treatment of patients with head and neck cancer. Cancer 75(2):569–576
Bjordal K, Kaasa S (1995) Psychological distress in head and neck cancer patients 7–11 years after curative treatment. Br J Cancer 71(3):592–597
Hatsukami DK, Stead LF, Gupta PC (2008) Tobacco addiction. Lancet 371(9629):2027–2038. doi:10.1016/S0140-6736(08)60871-5
Cinciripini PM, Hecht SS, Henningfield JE, Manley MW, Kramer BS (1997) Tobacco addiction: implications for treatment and cancer prevention. J Natl Cancer Inst 89(24):1852–1867
Cox LS, Africano NL, Tercyak KP, Taylor KL (2003) Nicotine dependence treatment for patients with cancer. Cancer 98(3):632–644. doi:10.1002/cncr.11538
Browman GP, Wong G, Hodson I, Sathya J, Russell R, McAlpine L, Skingley P, Levine MN (1993) Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med 328(3):159–163. doi:10.1056/NEJM199301213280302
Bandura A (2004) Health promotion by social cognitive means. Health Educ Behav 31(2):143–164. doi:10.1177/1090198104263660
Prochaska JO, DiClemente CC, Velicer WF, Rossi JS (1993) Standardized, individualized, interactive, and personalized self-help programs for smoking cessation. Health Psychol 12(5):399–405
Fiore MC (2000) Treating tobacco use and dependence: an introduction to the US Public Health Service Clinical Practice Guideline. Respir Care 45(10):1196–1199
Hakkaart-van Roijen L, Tan SS, Bouwmans CAM (2010) Manual for cost analyses, methods and standard prices for economic evaluations in health care (in Dutch). Amstelveen, The Netherlands
Pharmacotherapeutic Compass (in Dutch) (2008) Amstelveen, The Netherlands
Gritz ER, Carr CR, Rapkin D, Abemayor E, Chang LJ, Wong WK, Belin TR, Calcaterra T, Robbins KT, Chonkich G et al (1993) Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev 2(3):261–270
Simon JA, Carmody TP, Hudes ES, Snyder E, Murray J (2003) Intensive smoking cessation counseling versus minimal counseling among hospitalized smokers treated with transdermal nicotine replacement: a randomized trial. Am J Med 114(7):555–562
Ackerstaff AH, Hilgers FJ, Aaronson NK, Balm AJ (1994) Communication, functional disorders and lifestyle changes after total laryngectomy. Clin Otolaryngol Allied Sci 19(4):295–300
Godtfredsen NS, Prescott E, Osler M (2005) Effect of smoking reduction on lung cancer risk. Jama 294(12):1505–1510. doi:10.1001/jama.294.12.1505
Wewers ME, Jenkins L, Mignery T (1997) A nurse-managed smoking cessation intervention during diagnostic testing for lung cancer. Oncol Nurs Forum 24(8):1419–1422
Rigotti NA, Munafo MR, Stead LF (2008) Smoking cessation interventions for hospitalized smokers: a systematic review. Arch Intern Med 168(18):1950–1960. doi:10.1001/archinte.168.18.1950
Chan Y, Irish JC, Wood SJ, Sommer DD, Brown DH, Gullane PJ, O’Sullivan B, Lockwood GA (2004) Smoking cessation in patients diagnosed with head and neck cancer. J Otolaryngol 33(2):75–81
Sciamanna CN, Stillman FA, Hoch JS, Butler JH, Gass KG, Ford DE (2000) Opportunities for improving inpatient smoking cessation programs: a community hospital experience. Prev Med 30(6):496–503. doi:10.1006/pmed.2000.0668
Feenstra TL, Hamberg-van Rheenen HH, Hoogenveen RT, Rutten-van Molken MP (2005) Cost-effectiveness of face-to-face smoking cessation interventions: a dynamic modeling study. Value health 8:178–190
Hoogendoorn M, Feenstra TL, Hoogenveen RT, Rutten-van Molken MP (2010) Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD. Thorax 65(8):711–718. doi:10.1136/thx.2009.131631
Acknowledgments
We thank our oncology nurses from the outpatient clinic, Joke de Bakker, Carina Laros and Marianne Groot. We also want to thank Harm van Tinteren for helping with the statistical analysis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
de Bruin-Visser, J.C., Ackerstaff, A.H., Rehorst, H. et al. Integration of a smoking cessation program in the treatment protocol for patients with head and neck and lung cancer. Eur Arch Otorhinolaryngol 269, 659–665 (2012). https://doi.org/10.1007/s00405-011-1673-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-011-1673-0