Abstract
The aim of the study was to determine the prevalence and genotypes of HPV infection in laryngeal cancer specimens, normal mucosa obtained from the surgical margin and laryngeal nodules using a novel high sensitive and specific SPF10 HPV DNA test, PCR/DEIA method and INNO-LiPA genotyping assay. The correlation between HPV presence and clinico-pathological features was analyzed. Tissue samples were collected from 93 primary laryngeal squamous cell carcinoma (LSCC), 49 specimens of normal mucosa and from 22 specimens of laryngeal nodules serving as control group. HPV DNA was amplified by the short PCR fragment (SPF10) primer set using HPV DNA enzyme immunoassay (DNA/DEIA) method and INNO-LiPA HPV genotyping assay. Human papillomavirus was detected in 33 (35.5%) of the 93 samples from LSCC, in 4 (8.2%) of 49 samples of the normal mucosa and it was not detected in any of the sample from the control group. Twenty-eight of 33 (81.8%) were positive for HPV-16, 6 of 33 (18.2%) were positive for HPV-18 and 5 of 33 (15.1%) were positive for HPV-33. Multiple infection was found in 5 of 33 (15.1%); 3 samples were positive for HPV-16 and HPV-33, 2 samples for HPV-16 and HPV-18. There was a statistically significant correlation between the presence of HPV in LSCC tumors and in control group samples and between the presence of HPV in the tumors and normal mucosa from the free surgical margin. The presence of HPV infection in 35.5% of the cases suggests a possible role in the etiology of laryngeal cancer and supports the role of high-risk types of HPV (16, 18 and 33) in LSCC. HPV infection is not likely to influence survival rates as an independent prognostic factor in patients with laryngeal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The larynx is the most common site of malignancy in the upper aerodigestive tract and the laryngeal squamous cell carcinoma (LSCC) is the histology of the tumor in over than 90% of cases. The etiology of LSCC is considered to be multifactorial. The main predisposing factors are tobacco and alcohol use [1]. Human papillomavirus (HPV) infections, nutritional deficiency and local dietary customs have also been implicated in the etiology of laryngeal cancer [1–3].
Human papillomaviruses (HPVs) are small double-stranded DNA viruses that induce hyperproliferative lesions of cutaneous and mucosal epithelia. Papillomaviruses can be divided into low-risk types, which induce only benign lesions, and high-risk types, which are associated with the development of malignant lesions [4]. More than 90% of cervical cancers contain HPV DNA of the high-risk types [5, 6]. The two transforming proteins encoded by the high-risk HPVs, E6 and E7, function through their associations with the tumor suppressor proteins p53 and Rb [7]. One hundred and eighteen papillomavirus (PV) types have been completely described and approximately 120 additional isolates represent only partially characterized putative novel genotypes [5]. Particularly, HPV-16 is suspected to play a role in etiopathogenesis of LSCC [1, 2]. The diagnosis of HPV infection is almost entirely based on molecular tools, which are mainly PCR-based. General or consensus PCR primers have been developed that detect a broad spectrum of HPV genotypes in a single PCR [7–9]. Several techniques have been used to detect the presence of HPV in tissues. In LSCC a novel, high sensitive and broad-spectrum SPF10 PCR/DEIA method has been poorly investigated so far. The SPF10 PCR/DEIA method has been established and investigated in the cervical squamous cell carcinoma [8, 9].
The aim of the study was to determine the prevalence and genotypes of HPV infection in laryngeal cancer specimens, normal mucosa obtained from the surgical margin and laryngeal nodules using a novel high sensitive and specific SPF10 HPV DNA test and PCR/DEIA method. The correlation between the presence of HPV and clinico-pathological features was analyzed.
Materials and methods
Tissue specimens were obtained from 93 consecutive patients with primary LSCC operated upon by the first author in the Department of Otolaryngology Head and Neck Surgery, Medical University of Lublin in the years 1999–2002. The group consisted of 78 males and 15 females, aged between 32 and 78 years (mean 57.7 years). The specimens were collected during a primary diagnostic microlaryngoscopy. Patients were treated surgically with or without postoperative radiotherapy depending of the clinical stage of the disease. The patients had neither previous radiotherapy nor chemotherapy. TNM classification was according to the Union Against Cancer (UICC) criteria. Histologic grading was performed according to the World Health Organization criteria, which divides the tumor into well differentiated (G1), moderately well differentiated (G2), and poorly differentiated (G3) types.
Tissue samples from the normal mucosa from the surgical margin were collected from 49 patients with LSCC. After that, total laryngectomy samples of the normal mucosa from the surgical margin, 1–2 cm from the palpated or visible tumor, were obtained. Special care was taken not to contaminate the samples. Single use and sterile instruments were used to harvest and store the samples. The lymph-node metastases were not tested for HPV presence. Non-neoplastic laryngeal lesions -laryngeal nodules served as control group. The tissue specimens were harvested from 22 patients, 12 males and 10 females aged between 21 and 71 years (mean 41.4 years) operated in our institution and the histological evaluation confirmed the diagnosis in all cases.
The clinicopathological features of the patients with LSCC are shown in Table 1.
The study project was approved by the Local Institutional Review Board.
DNA extraction from paraffin sections
Five, 10-μm sections of formalin-fixed, paraffin-embedded tissue were transferred to eppendorf vials after cutting deep into the block. The microtome blade was changed after each case. DNA extraction from tissue specimens was done using standardization Genomic Mini test for DNA extraction kit (Genomic DNA Prep Plus A&A Biotechnology Gdynia, Poland). After deparaffinization, tissue sample was transferred to fresh eppendorf viols and incubated at 50°C with 100 μl Tris buffer, 50 μl universal lyses solution LT and 20 μl proteinase K, and was rotated for the complete digesting of the tissue sample. After incubation, 150 μl LT buffer were added and it was centrifuged for 3 min. at 10,000–15,000 rpm and washed twice with 98% ethanol. The precipitated DNA was centrifuged for 1 min at 10,000–15,000 rpm and again washed with 70% ethanol. The DNA was precipitated and dried. The dried DNA extracts were incubated for 5 min at room temperature with 200 μl Tris buffer (10 mM Tris–HCl pH 8.5), which had a 75°C temperature and then it was centrifuged for 1 min at 10,000–15,000 rpm. The obtained DNA extracts was stored at −25°C for further analysis.
β-globin amplification
To analyze the DNA quality PCR amplification of β-globin was performed in a separate reaction using primers PC04 and KM29 with fragment of 205 bp. The sequence of the set primer of PCO4 was d(5′-CAA CTT CAT CCA CGT TCA CC-3′) and for KM29 was d(5′-GGT TGG CCA ATC TAC TCC CAG G-3′).
HPV DNA detection
Human papillomavirus DNA was amplified by the short PCR fragment (SPF10) HPV primer set (Labo Bio-medical Products B.V., Holland). The SPF10 primers amplify a 65-bp fragment from the L1 region of the HPV genome as described by Kleter et al. [8]. The PCR products were analyzed by both 3% agarose gel electrophoresis and HPV DNA enzyme immunoassay (DNA/DEIA). Each run was accompanied by quality control samples. During each PCR run, samples were tested, together with one negative control (distilled H2O) and one positive control (Caski culture cell line). Amplification products were tested by probe hybridization in a microtiter plate assay to detect the presence of HPV DNA as described earlier by Kleter et al. [8] This assay also included appropriate negative and positive controls. Amplicons from HPV-positive samples were analyzed using the INNO-LiPA HPV genotyping assay, which permits specific detection of 25 HPV genotypes (HPV types 6, 11, 16, 18, 31, 33, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68, 70, 73 and 74). The assay has been described in detail earlier [8, 9].
Control samples
DNA from the Caski cell line was used as a positive PCR control to assess the success of the amplification. PCR reagents lacking DNA (Distilled H2O) served in each PCR amplification as a negative control. The detection of DNA of necessary quality was performed by β-globin assay.
Statistical analysis
Statistical analysis was performed to investigate the relationship between HPV presence and clinico-pathological and demographical parameters by means of the χ2 test (2 × 2 table and contingence table). Survival rates at 3 and 5-year follow-up for overall survival (OS) and disease-specific survival (DSS) were calculated using the method of Kaplan–Meier and the differences between curves were assessed with the log-rank test. Cox’s regression model was used to evaluate the predictive power of various factors in multivariate analysis. Statistical significance was defined as P < 0.05.
Results
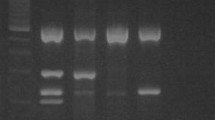
The β-globin DNA was amplified in 93 samples with LSCC (Fig. 1), in 49 samples of normal mucosa from the larynx and in 22 patients with laryngeal nodules indicating the presence of amplifiable DNA in all cases.
Using the SPF10 set primer HPV was detected in 33 (35.5%) of the 93 samples from LSCC (Fig. 2), in 4 (8.2%) of 49 samples of the normal mucosa from the surgical margin in patients with LSCC and it was not detected in any of the sample from the control group. In patients with the HPV-positive samples in the normal mucosa from the surgical margin, the tumor samples were also HPV positive. Among the HPV-positive samples from LSCC, 28 of 33 (81.8%) were positive for HPV-16, 6 of 33 (18.2%) were positive for HPV-18 and 5 of 33 (15.1%) were positive for HPV-33. Multiple infection was found in 5 of 33 (15.1%); 3 samples were positive for HPV-16 and HPV-33, 2 samples for HPV-16 and HPV-18. Among the HPV-positive samples from normal mucosa from the surgical margin in patients with LSCC all four samples were positive for HPV-16.
The difference between the presence of HPV in squamous cell carcinoma tumors and in control group samples was statistically significant (χ2 = 8.13; P = 0.004). The difference between the presence of HPV in squamous cell carcinoma tumors and in normal mucosa from the surgical margin in patients with LSCC samples was statistically significant (χ2 = 3.78; P = 0.05).
The demographical and clinicopathological features of HPV positive patients are shown in Table 1. Twenty-seven males and 6 females were HPV positive. Eight (24.2%) of 33 samples with HPV positive tumors were classified as G1, 19 (57.6%) were G2 and 6 (18.2%) were G3. Twenty (60.6%) of the 33 HPV positive tumors were supraglottic, 12 (36.4%) were located in glottis and one (3%) in subglottic region. Twenty-eight (84.4%) of the 33 HPV positive tumors were T3 or T4 and 5 (15.6%)were T1 or T2. Fifteen (45.5%) of 33 HPV positive patients had no clinically evident cervical lymph nodes (N0) and 18 (54.5%) of HPV positive patients had lymph node involvement (N1 or N2). Twenty-six HPV-positive patients were heavy cigarette smokers and 23 of 33 frequently consumed alcohol. No significant correlation was found between HPV incidence and epidemiological factors or histological grade or clinical stage of tumor.
Two males and two females were HPV positive. Three of the four HPV-positive tumors were supraglottic and one (25%) was located in glottis. In all of the patients with HPV-positive normal mucosa the tumors were in the advanced stage (T3 or T4). One of the four patients with HPV-positive normal mucosa had no clinically evident cervical lymph nodes (N0) and three patients with HPV-positive normal mucosa had evident lymph-node involvement. In three of four patients with HPV-positive normal mucosa, tumors were G3 and in 1 sample the tumor was G1 (well differentiated).
Survival and recurrence analysis
It was possible to calculate the 3-year survival rates in 93 and the 5-year survival in 79 patients. Both OS and DSS were analyzed. The three-year survival data were available for 93 patients. Thirty-five deaths, including 28 from laryngeal cancer and 7 from unrelated causes, occurred in the study population at 3 years of follow-up. Thirty-eight deaths, including 23 from laryngeal cancer and 15 from unrelated cases, occurred in the study population at 5 years of follow-up.
From the whole group of 93 patients with LSCC, 58 (62.4%) were alive at last follow-up 3 years after initial treatment for OS and 58 (67.4%) of 86 for DSS. The difference in the 3-year survival rates between the groups of HPV positive versus HPV-negative patients was not significant for OS (P = 0, 4) and DSS (P = 0.77).
In 26 patients from the whole group of 93 patients there was recurrence of local or regional nodes or local plus regional nodes. From the 33 HPV-positive patients, 11 (33.3%) developed a local or regional nodes or local plus regional node recurrence. There was no significant correlation between the presence of HPV and recurrence rates at 3 years of follow up (χ2 = 0.73; P = 0.39).
From the 28 HPV-positive patients, 13 (46.4%) patients were alive at the last 5 years of follow-up and 15 (53.6%) patients died for OS. From the 24 HPV positive patients, 13 (54.2%) patients were alive for DSS. From the whole group of 79 patients with LSCC 41 (51.9%) were alive at the last 5 years of follow-up after initial treatment for OS and 41 (58.6%) of 70 patients for DSS. The difference in the 5-year survival rates between the groups of HPV positive and HPV-negative patients was not significant for OS (P = 0.66) nor for DSS (0.49).
In 27 patients from the whole group of 79 patients local or regional node recurrence or local plus regional node recurrence was observed at the 5-year follow-up. From the 28 HPV-positive patients, 11 (39.3%) patients developed a local or regional node recurrence or local plus regional node recurrence. There was no significant correlation between the presence of HPV and recurrence rates at the 5-years follow up (χ2 = 0.5; P = 0.48).
From the whole group of 49 patients with LSCC and normal mucosa assay, 33 (67.3%) were alive at the last follow-up of 3 years after initial treatment for OS. The difference in the 3-year survival rates between the groups of HPV positive and HPV-negative patients was not significant (P = 0.83). In 15 patients from the whole group of 49 patients local or regional nodes or local plus regional node recurrence was observed. From the four HPV-positive patients, two (50%) developed a local recurrence. There was no significant difference between the HPV presence and recurrence at the 3-year follow-up (χ2 = 0.1; P = 0.76).
From the group of 36 patients with normal mucosa samples 18 (50%) were alive at the last follow-up 5 years after the initial treatment for OS. The difference in the 5-year survival rates between the groups of HPV positive and HPV-negative patients was not significant (P = 0.6). From the three patients with HPV-positive mucosa sample, two patients developed local recurrence. There was no significant difference between the HPV presence and recurrence at 5 years of follow-up (χ2 = 0.17; P = 0.68). The statistical analysis of the presence of HPV in squamous cell carcinoma tumors and normal mucosa was not performed because between the HPV presence and recurrence at the 3-year follow-up the group of HPV-positive mucosa specimens was too small for reliable evaluation.
A multivariate analysis of survival applying the Cox regression model showed that the HPV presence in tumor samples or normal mucosa is not an independent prognostic factor.
Discussion
Recently, several techniques have been used to detect the presence of HPV in tissues, for example Southern-blot analysis, previously considered to be the most sensitive assay or in situ hybridization analysis, which can be performed on paraffin-embedded sections. Those techniques have the disadvantage of requiring a sizable quantity of nucleic acids and take several days to perform. PCR is more sensitive than other techniques but its high sensitivity may lead to product carryover or DNA contamination, which can create problems in diagnostic applications.
DNA amplification methods, such as the PCR, permit more sensitive detection of viral DNA. Except type-specific PCR primers for individual HPV genotypes, several universal PCR primer sets have been developed, including My11/My09, OB/II, CPI/CPII, and GP5+/6+. However, it has been shown that none of these general primer sets permits adequate detection of the still expanding spectrum of HPV genotypes. The sensitivity of these universal primer sets is limited and their use will underestimate the true prevalence of HPV. The SPF10 PCR/DEIA method was developed as a set of HPV PCR primers for the highly sensitive detection of all known mucosal HPV types.
Kleter et al. [8] showed that the detection rate in 83.7% was significantly higher using the SPF system as compared with the universal primer set GP5+/6+. Kleter et al. [8] also reported that the SPF assay detected HPV DNA in 100% of 184 formalin-fixed, paraffin-embedded cervical carcinoma specimens. Kleter et al. [8] reported that the novel SPF system permitted universal and highly sensitive detection of HPV DNA in diverse clinical materials and may improve the molecular diagnosis and epidemiology of this important virus.
A novel, high sensitive and broad-spectrum SPF10 PCR/DEIA method has been established and investigated in the cervical squamous cell carcinoma [8, 9]; however, it is poorly investigated in laryngeal cancer. The reported frequency of HPV DNA in LSCC varies between 3 and 60%, depending on tumor site, type of specimens and methods applied for analysis, as well as the number of cases included [2, 3, 10–12]. Some authors claim that HPV infection plays a possible role in the etiology of LSCC [2, 3, 11, 12] others do not prove this correlation [10].
To our knowledge, this is the first report that has applied SPF10 PCR primer set and PCR/DEIA and genotypes in a big group with LSCC. HPV DNA was determined in 35.5% of 93 paraffin-embedded tissue samples of LSCC. Koskinen et al. [13], analyzed the incidence of HPV DNA presence and genotypes using SPF10 PCR screening with a general probe hybridization and INNO-LiPA HPV genotyping assay in head and neck squamous cell carcinoma. Thirty-seven of 61 (61%) samples were HPV positive. HPV-16 was the most frequently detected type and it was present in 31 of 37 HPV positive samples. They detected multiple type infections in 8 of 37 (22%) of the HPV-positive samples and co-infection by HPV-16 and HPV-33 was predominant [13]. In the group analyzed by Koskinen et al. [13], the material was obtained from variety of primary tumors. They included hypopharynx, larynx, tongue, oral cavity and tonsil carcinoma samples. The incidence of HPV infection was higher in patients with tonsil and oral cavity carcinoma. Pirog et al. [14] using the SPF 10 primer set and line probe assay (LiPA) method demonstrated the presence of HPV DNA in 82 of 90 (91%) patients with mucinous adenocarcinomas, and in all 9 adenosquamous tumors (100%). The most common viral types detected in adenocarcinoma were HPV16 (50%) and HPV 18 (40%), followed by HPV 45 (10%), HPV 52 (2%), and HPV 35 (1%). Multiple HPV types were detected in 9.7% of the cases. Almadori et al. [15] using PCR technique with HPV consensus primers, detected HPV DNA in 15 of the 42 (35.7%) tumors, and it belonged almost exclusively to the highly oncogenic HPV-16, HPV-18, and HPV-33 genotypes. In 1996, Almadori et al. [16] detected HPV DNA in 9 of 45 (20%) patients using PCR technique. They also showed that in 55% of the patients, the HPV-positive tumors were G2 and in 33% G3. Fifty-five percent of the HPV positive had no clinical cervical lymph nodes (N0). It was observed that 78% of the HPV positive cancer patients were T3–T4. Almadori et al. [16] described more HPV-positive patients with supraglottic tumors than with tumors in other locations. Hoshikawa et al. [17] detected HPV DNA in 17.6% of laryngeal cancer patients by PCR. Glottic laryngeal carcinoma was HPV positive in 44.4% cases and it was significantly higher than 8.7% HPV positive supraglottic tumors. They suggested that HPV infection might be more frequent in the vocal cord tumors than in other laryngeal sites. The data also showed that HPV-16 was the most frequently detected type and it was present in 27 of 33 HPV-positive samples and multiple type infections were detected in 5 of 33 (15.1%) of the HPV-positive samples. The results support the role of high-risk types of HPV in LSCC.
The data in the present study showed that the HPV-positive tumors were more frequently located in supraglottis than in glottic region, but patients with supraglottic tumors were the majority of the whole group and statistical analysis revealed no significant differences in the incidence of HPV and localization of the tumor.
Some authors claim that HPV DNA is more frequently detected in well differentiated [18, 19], than in poorly differentiated squamous cell carcinoma of the upper aerodigastive tract [20]. In this study, HPV was detected more often in well differentiated (63.3%) and moderately (20%) differentiated than in poorly (16.7%) differentiated tumors. Still, the differences were not statistically significant. Gillison et al. [21] demonstrated that HPV-positive head and neck squamous cell carcinomas (HNSCCs) were not statistically significantly different from HPV-negative HNSCCs with regard to the well-established risk factors of alcohol consumption and tobacco exposure. Similar results were obtained in this study. Gillison et al. [21] found that HPV-positive HNSCC patients had significantly improved DSS when compared with HPV-negative tumors, even after adjustment for age, lymph node status, and heavy alcohol consumption. The improved DSS survival in patients with HPV-positive HNSCC reported by Gillison et al. [21] is somewhat surprising and remains unexplained.
In the present study HPV-positive LSCC patients had no significantly improved OS and DSS survival when compared with patients with HPV-negative tumors. Gillison et al. [21] suggested that patients with HPV-positive tumors might be less susceptible to the development of synchronous or metasynchronous tumors in the lungs, esophagus, and elsewhere in the head and neck that could adversely affect the long-term survival. The HPV-positive tumors may be less associated with alcohol and tobacco exposure and HPV infections tend to be focal, filed cancerization in which the upper respiratory epithelium is repeatedly exposed to carcinogens my be less applicable [22].
In the present study HPV DNA infection in normal laryngeal mucosa from the surgical margin in patients with LSCC was detected in 8.2% of 49 normal mucosa samples but none of the samples from control group was HPV DNA positive. A prevalence of 4% of HPV DNA infection in normal laryngeal mucosa of healthy patients has been reported by Brandsma et al. [23]. Nunez et al. [24] determined the prevalence of HPV in a series of normal laryngeal mucosa. Twelve autopsy larynges were collected. Evidence of HPV infection was documented by the PCR using oligonucleotide primers complementary to sequences in the E6 region of HPV types 11, 16 and 18. HPV type 11 was isolated from three specimens. A 25% prevalence rate for HPV 11 was found. No other HPV types were isolated [24]. The presence of HPV infection in the premalignant and hyperplastic laryngeal lesions is controversial. Poljak et al. [25] analyzed the prevalence of HPV in laryngeal epithelial hyperplastic lesions using PCR and in situ hybridization methods. HPV was present in only 2 of 88 specimens and the authors suggested that most of hyperplastic lesions in the larynx are not associated with HPV infection.
Conclusion
The presence of HPV infection in 35.5% of the cases suggests a possible role in the etiology of laryngeal cancer and supports the role of high-risk types of HPV (16, 18 and 33) in LSCC. The incidence of HPV infection in patients with LSCC tumor was significantly higher than in control group and higher than in normal mucosa from the surgical margin in patients with LSCC samples.
HPV infection is not likely to influence survival rates as an independent prognostic factor in patients with laryngeal cancer. The high sensitive and specific SPF10 HPV DNA test, PCR/DEIA method and INNO-LiPA genotyping assay can be performed for HPV detection on paraffin-embedded sections collected during diagnostic procedures as good screening test.
References
Maier H, Dietz A, Gewelka U, Heller WD, Weidauer H (1992) Tobacco and alcohol and the risk of head and cancer. Clin Invest 70:320–327
Morshed K, Stenzel A, Szymański M, Różyńska K, Siwiec H, Gołąbek W, Wojcierowski J (2001) Detection of human papillomavirus typ 16 and 18 in laryngeal cancer using PCR. Otolaryngol Pol 55:29–33
Morshed K, Korobowicz E, Szymanski M, Skomra D, Golabek W (2005) Immunohistochemical demonstration of multiple HPV types in laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 262:917–920
Howley PM (1996) Papillomavirinae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM (eds) Fundamental virology, 3rd edn. Lippincott-Raven, Philadelphia, pp 947–978
de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H (2004) Classification of papillomaviruses. Virology 324:17–27
Lowy DR, Kirnbauer R, Schiller JT (1994) Genital human papillomavirus infection. Proc Natl Acad Sci USA 91:2436–2440
Jacobs MV, Snijders PJ, van den Brule AJ, Helmerhorst TJ, Meijer CJ, Walboomers JM (1997) A general primer GP5+/GP6+-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol 35:791–795
Kleter B, van Doorn LJ, ter Schegget J, Schrauwen L, van Krimpen K, Burger M, ter Harmsel B, Quint W (1998) A novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol 153:1731–1739
Kleter B, van Doorn LJ, Schrauwen L, Molijn A, Sastrowijoto S, ter Schegget J, Lindeman J, ter Harmsel B, Burger M, Quint W (1999) Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol 37:2508–2517
Brandwein MS, Nuovo GJ, Biller H (1993) Analysis, of prevalence of human papillomavirus in laryngeal carcinomas. Study of 40 cases using polymerase chain reaction and consensus primers. Ann Otol Rhinol 102:309–313
Garcia-Milian R, Hernandez H, Panade L, Rodrriguez C, Gonzalez N, Valenzuela C, Arana MD, Perea SE (1998) Detection and typing of human papillomavirus in benign and malignant tumours of laryngeal epithelium. Acta Otolaryngol 118:754–758
Venuti A, Manni V, Morello R, De Marco F, Marzetti F, Marcante ML (2000) Physical state and expression of human papillomavirus in laryngeal carcinoma and surrounding normal mucosa. J Med Virol 60:396–402
Koskinen WJ, Chen RW, Leivo I, Makitie A, Back L, Kontio R, Suuronen R, Lindqvist C, Auvinen E, Molijn A, Quint WG, Vaheri A, Aaltonen LM (2003) Prevalence and physical status of human papillomavirus in squamous cell carcinomas of the head and neck. Int J Cancer 107:401–406
Pirog EC, Kleter B, Olgac S, Bobkiewicz P, Lindeman J, Quint WG, Richart RM, Isacson C (2000) Prevalence of human papillomavirus DNA in different histological subtypes of cervical adenocarcinoma. Am J Pathol 157:1055–1062
Almadori G, Cadoni G, Cattani P, Galli J, Bussu F, Ferrandina G, Scambia G, Fadda G, Maurizi M (2001) Human papillomavirus infection and epidermal growth factor receptor expression in primary laryngeal squamous cell carcinoma. Clin Cancer Res 7:3988–3993
Almadori G, Cadoni G, Cattani P, Posteraro P, Scarano E, Ottaviani F, Paludetti G, Maurizi M (1996) Detection of human papillomavirus DNA in laryngeal squamous cell carcinoma by polymerase chain reaction. Eur J Cancer 32A:783–788
Hoshikawa T, Nakajima T, Uhara H, Gotoh M, Shimosato Y, Tsutsumi K, Ono I, Ebihara S (1990) Detection of human papillomavirus DNA in laryngeal squamous cell carcinomas by polymerase chain reaction. Laryngoscope 100:647–650
Anwar K, Nakakuki K, Naiki H, Inuzuka M (1993) ras gene mutations and HPV infection are common in human laryngeal carcinoma. Int J Cancer 2:22–28
Ishibashi T, Matsushima S, Tsunokawa Y, Asai M, Nomura Y, Sugimura T, Terada M (1990) Human papillomavirus DNA in squamous cell carcinoma of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg 116:294–298
Perez-Ayala M, Ruiz-Cabello F, Esteban F, Concha A, Redondo M, Oliva MR, Cabrera T, Garrido F (1990) Presence of HPV 16 sequences in laryngeal carcinomas. Int J Cancer 46:8–11
Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92:709–720
Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, Corio R, Lee D, Greenberg B, Koch W, Sidransky D (1996) Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res 56:2488–2492
Brandsma JL, Abramson AL (1989) Association of papillomavirus with cancer of the head and neck. Arch Otolaryngol Head Neck Surg 115:621–625
Nunez DA, Astley SM, Lewis FA, Wells M (1994) Human papilloma viruses: a study of their prevalence in the normal larynx. J Laryngol Otol 108:319–320
Poljak M, Gale N, Kambic V (1997) Human papillomaviruses: a study of their prevalence in the epithelial hyperplastic lesions of the larynx. Acta Otolaryngol Suppl 527:66–69
Acknowledgments
This project is supported by grant of Polish State Committee for Scientific Research (nr 3 P05C 06224). I would like to thank Dr B. Rajtar from Virology Department, Medical University of Lublin, Poland for his help in sample preparation and Prof W. Gołąbek from Department of Otolaryngology, Head and Neck Surgery for the advice and help in study design. Dr Agata Smoleń from Department of Mathematics and Biostatistics, Medical University of Lublin is thanked for the help in statistical analysis of the presented data.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Morshed, K., Polz-Dacewicz, M., Szymański, M. et al. Short-fragment PCR assay for highly sensitive broad-spectrum detection of human papillomaviruses in laryngeal squamous cell carcinoma and normal mucosa: clinico-pathological evaluation. Eur Arch Otorhinolaryngol 265 (Suppl 1), 89–96 (2008). https://doi.org/10.1007/s00405-007-0569-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-007-0569-5