Abstract
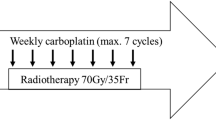
Unresectable head and neck squamous cell carcinoma (HNSCC), non-metastatic, comprises a heterogeneous group of patients (pts), formed of stage III and IV pts. Since the available literature had not distinguished among these two groups, we prospectively addressed whether the recommended regimen involving cisplatin 100 mg/m2 concurrent to conventionally delivered radiotherapy (RT) is feasible in stage IV pts, based on the efficacy and safety of this regimen. A total of 30 pts were enrolled onto this study. Chemoradiation (CRT) consisted of RT 70 Gy, delivered in 35 daily fractions of 2 Gy, in 7 weeks, concurrent to cisplatin 100 mg/m2 on days 1, 22 and 43. Supportive treatment was provided as needed. Twenty-eight pts had tumors staged as T4 and 20 had N2 or N3 cervical involvement. The most common primary sites were the oral cavity and the oropharynx (23 pts). We observed six complete responses and 12 partial responses, with an overall response rate of 60%. A high rate of treatment-related toxicities was observed, with three deaths during CRT, and 26 pts suffering from one or more grade 3/4 toxicities, mainly dysphagia, mucositis, dermatitis, vomiting, infection or anemia. A prolonged treatment time was observed (63 days), as a result of unplanned treatment breaks. The lack of requirement of red blood cell transfusion was favorably related to the response to the treatment (93% vs. 50%, P = 0.033). For the whole population, with a median follow-up of 20.8 months, the median progression-free survival (PFS) was 8.0 months, and the median overall survival (OS) was 17.3 months. Longer median PFS and OS were seen in responding pts (12.8 vs. 4.1 months, P = 0.0001; and not reached (NR) vs. 10.4 months, P = 0.0037, respectively), as well as in those pts not requiring red blood cell transfusion (12.8 vs. 3.9 months, P = 0.0162; and NR vs. 10.4 months, P = 0.0176, respectively). In conclusion, this concurrent CRT regimen is hardly delivered in stage IV, unresectable, locally advanced HNSCC pts, due to treatment-related toxicities and longer RT duration. As a subset of pts may benefit from this regimen, adequate patient selection and aggressive supportive measures are essential.

Similar content being viewed by others
References
Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF, Schuller DE, Forastiere AA (2003) An Intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98
Cooper JS, Farnan NC, Asbell SO, Rotman M, Marcial V, Fu KK, McKenna WG, Emami B (1996) Recursive partitioning analysis of 2105 patients treated in Radiation Therapy Oncology Group studies of head and neck cancer. Cancer 77:1905–1911
Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M (2002) AJCC cancer staging handbook. 6th edn. Springer, New York
Pignon JP, Bourhis J, Domenge C, Designé L (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet 355:949–955
Available at http://www.fosp.saude.sp.gov.br, accessed in June 23rd, 2006
Bourhis J, Amand C, Pignon JP (2004) Update of MACH-NC (Meta-analysis of chemotherapy in head and neck cancer) database focused on concomitant chemoradiotherapy. J Clin Oncol 22(Suppl 14):5505
Rosenthal DI, Ang KK (2004) Altered radiation therapy fractionation, chemoradiation, and patient selection for the treatment of head and neck squamous carcinoma. Semin Radiat Oncol 14:153–166
Brizel DM, Albers ME, Fisher SR, Scher RL, Richtsmeier WJ, Hars V, George SL, Huang AT, Prosnitz LR (1998) Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med 338:1798–1804
Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Calais G (2004) Final results of the 94–01 French head and neck oncology and radiotherapy group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 22:69–76
Huguenin P, Beer KT, Allal A, Rufibach K, Friedli C, Davis JB, Pestalozzi B, Schmid S, Thoni A, Ozsahin M, Bernier J, Topfer M, Kann R, Meier UR, Thum P, Bieri S, Notter M, Lombriser N, Glanzmann C (2004) Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol 22:4665–4673
Jeremic B, Shibamoto Y, Milicic B, Nikolic N, Dagovic A, Aleksandrovic J, Vaskovic Z, Tadic L (2000) Hyperfractionated radiation therapy with or without concurrent low-dose daily cisplatin in locally advanced squamous cell carcinoma of the head and neck: a prospective randomized trial. J Clin Oncol 18:1458–1464
Merlano M, Benasso M, Corvò R, Rosso R, Vitale V, Blengio F, Numico G, Margarino G, Bonelli L, Santi L (1996) Five-year update of a randomized trial of alternating radiotherapy and chemotherapy compared with radiotherapy alone in treatment of unresectable squamous cell carcinoma of the head and neck. J Natl Cancer Inst 88:583–589
Wendt TG, Grabenbauer GG, Rödel CM, Thiel HJ, Aydin H, Rohloff R, Wustrow TPU, Iro H, Papella C, Schalhorn A (1998) Simultaneous radiochemotherapy versus radiotherapy alone in advanced head and neck cancer: a randomized multicenter study. J Clin Oncol 16:1318–1324
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113:9–19
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Suntharalingam M, Haas ML, Van Echo DA, Haddad R, Jacobs MC, Levy S, Gray WC, Ord RA, Conley BA (2001) Predictors of response and survival after concurrent chemotherapy and radiation for locally advanced squamous cell carcinomas of the head and neck. Cancer 91:548–554
Vokes EE, Kies MS, Haraf DJ, Stenson K, List M, Humerickhouse R, Dolan ME, Pelzer H, Sulzen L, Witt ME, Hsieh YC, Mittal BB, Weichselbaum RR (2000) Concomitant chemoradiotherapy as primary therapy for locoregionally advanced head and neck cancer. J Clin Oncol 18:1652–1661
Eisbruch A, Normolle DP (2005) Testing new chemoradiation regimens for head-and-neck cancer. Int J Radiat Oncol Biol Phys 61:5–6
Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, Garden AS, Ridge JA, Cooper JS, Ang KK (2000) A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 48:7–16
Withers HR, Taylor JM, Maciejewski B (1988) The harzard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 27:131–146
Prosnitz RG, Yao B, Farrell CL, Clough R, Brizel DM (2005) Pretreatment anemia is correlated with the reduced effectiveness of radiation and concurrent chemotherapy in advanced head and neck cancer. Int J Radiat Oncol Biol Phys 61:1087–1095
Bourhis J, Le Maître A, Pignon J, Ang K, Bernier J, Overgaard J, Tobias J, Saunders M, Adelstein D, O’Sullivan B (2006) Impact of age on treatment effect in locally advanced head and neck cancer: two individual patient data meta-analyses. J Clin Oncol 24(Suppl 18):5501
Bentzen SM, Atasoy BM, Daley FM, Dische S, Richman PI, Saunders MI, Trott KR, Wilson GD (2005) Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol 23:5560–5567
Overgaard J, Eriksen JG, Nordsmark M, Alsner J, Horsman MR (2005) Plasma osteopontin, hypoxia, and response to the hypoxia sensitiser nimorazole in radiotherapy of head and neck cancer: results from the DAHANCA 5 randomised double-blind placebo-controlled trial. Lancet Oncol 6:757–764
Eriksen JG, Alsner J, Steiniche T, Overgaard J (2005) The possible role of TP53 mutation status in the treatment of squamous cell carcinomas of the head and neck (HNSCC) with radiotherapy with different overall treatment times. Radiother Oncol 76:135–142
de Castro G Junior, Federico MH (2006) Evaluation, prevention and management of radiotherapy-induced xerostomia in head and neck cancer patients. Curr Opin Oncol 18:266–270
Garden AS, Morrison WH, Rosenthal DI, Chao KS, Ang KK (2004) Target coverage for head and neck cancers treated with IMRT: review of clinical experiences. Semin Radiat Oncol 14:103–109
Brizel DM, Le QT, Rosenthal D, Meredith R, Brizel HE, Heard R, Yao B, Eng T, Sailer S, Chen Y, Murphy B, Mendenhall W (2002) Phase 2 study of recombinant human keratinocyte growth factor (rHuKGF) in head and neck cancer treated with standard or hyperfractionated irradiation and concurrent chemotherapy. Int J Radiat Oncol Biol Phys 54(Suppl):285–286
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100(Suppl 9):2026–2046
Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, Horiot JC, Le Maitre A, Pajak TF, Poulsen MG, O’Sullivan B, Dobrowsky W, Hliniak A, Skladowski K, Hay JH, Pinto LH, Fallai C, Fu KK, Sylvester R, Pignon JP (2006) Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 368:843–854
Horiot JC, Bontemps P, van den Bogaert W, Le Fur R, van den Weijngaert D, Bolla M, Bernier J, Lusinchi A, Stuschke M, Lopez-Torrecilla J, Begg AC, Pierart M, Collette L (1997) Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: results of the EORTC 22851 randomized trial. Radiother Oncol 44:111–121
Remenar E, Van Herpen C, Germa Lluch J, Stewart S, Gorlia T, Degardin M, Bernier J, Spirlet C, Vermorken JB (2006) A randomized phase III multicenter trial of neoadjuvant docetaxel plus cisplatin and 5-fluorouracil (TPF) versus neoadjuvant PF in patients with locally advanced unresectable squamous cell carcinoma of the head and neck. Final analysis of EORTC 24971. J Clin Oncol 24(Suppl 18):5516
Hitt R, Lopez-Pousa A, Martinez-Trufero J, Escrig V, Carles J, Rizo A, Isla D, Vega ME, Marti JL, Lobo F, Pastor P, Valenti V, Belon J, Sanchez MA, Chaib C, Pallares C, Anton A, Cervantes A, Paz-Ares L, Cortes-Funes H (2005) Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 23:8636–8645
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Overgaard J, Hansen HS, Overgaard M, Bastholt L, Berthelsen A, Specht L, Lindelov B, Jorgensen K (1998) A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) Protocol 5–85. Radiother Oncol 46:135–146
Rischin D, Peters L, Fisher R Macann A, Denham J, Poulsen M, Jackson M, Kenny L, Penniment M, Corry J, Lamb D, McClure B (2005) Tirapazamine, cisplatin, and radiation versus fluorouracil, cisplatin, and radiation in patients with locally advanced head and neck cancer: a randomized phase II trial of the Trans-Tasman Radiation Oncology Group (TROG 98.02). J Clin Oncol 23:79–87
Overgaard J, Hansen HS, Specht L, Overgaard M, Grau C, Andersen E, Bentzen J, Bastholt L, Hansen O, Johansen J, Andersen L, Evensen JF (2003) Five compared with six fractions per week of conventional radiotherapy of squamous-cell carcinoma of head and neck: DAHANCA 6 and 7 randomised controlled trial. Lancet 362:933–940
Overgaard J, Mohanti BK, Bhasker S, Begum N, Ali R, Agerwal J, Kuddu M, Baeza M, Vikram B, Grau C (2006) Accelerated versus conventional fractionated radiotherapy in squamous cell carcinoma of the head and neck. A randomized international multicenter trial with 908 patients conducted by the IAEA-ACC Study Group. Int J Radiat Oncol Biol Phys 66(Suppl):13
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Castro , G., Snitcovsky, I.M.L., Gebrim, E.M.M.S. et al. High-dose cisplatin concurrent to conventionally delivered radiotherapy is associated with unacceptable toxicity in unresectable, non-metastatic stage IV head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol 264, 1475–1482 (2007). https://doi.org/10.1007/s00405-007-0395-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-007-0395-9