Abstract
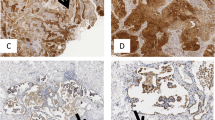
Head and neck cancers remain a big challenge for oncology. Among them laryngeal carcinomas predominate. In spite of abundant inflammatory cell infiltrates containing several immunologically competent cells, patients with head and neck cancers show markedly suppressed anti-tumor response. In general, cancer cells use strategies to avoid recognition and destruction by the immune system. Toll-like receptors 1–13 (TLRs) are crucial for activation of innate immunity and secondarily for the induction of acquired response. TLRs are mainly expressed on cells of the immune system, but they have been demonstrated on endothelial and epithelial cells. Ligand binding to TLR leads to the activation of several genes, predominantly proinflammatory ones such as IL-1 and TNF-alpha and maturation of professional antigen presenting cells (APC) i.e., dendritic cells. It can cause better tumor antigen presentation by APC. The aim of this study was the evaluation of expression of TLR-2, TLR-3 and TLR-4 in the microenvironment of laryngeal carcinoma. Tumor specimens (n = 20, male patients aged 43–77 years, mean 57 years) from patients subjected to total laryngectomy. Immunohistochemistry and indirect immunoflourescence on frozen tissue sections. Cancer tissue: portion of cancer cells manifested membrane and/or cytoplasmic expression of TLR-2, TLR-3 and TLR-4. The most frequent expression on tumor cells was TLR-2 and the least expression of TLR-4. Inflammatory infiltrates: in all cases inflammatory cell infiltrates of various intensities were present, both in tumor mass and tumor stroma. Expression of all TLRs tested, both, membrane and cytoplasmic ones were shown on inflammatory cells, but distinct in quantitative terms. TLR-4 positive cells were the most frequent. A portion of cells expressed both, TLR and HLA-DR. It is of interest that TLRs tested were expressed not only on cells of inflammatory infiltrate, but also on tumor cells. This fact may be an important factor in tumor escape from immune surveillance. It is notable, that both, TLRs and HLA-DR were shown to be co-expressed, what may favor the role and impact of TLRs in antigen presentation. Further studies are needed to elucidate TLRs function in the course of neoplastic process.





Similar content being viewed by others
References
Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK (2002) Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem 277:15028–15034
Baniyash M (2004) TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol 4:675–687
Barton GM, Medzhitov R (2002) Control of adaptive immune responses by toll-like receptors. Curr Opin Immunol 14:380–383
Brondani Da Rocha A, Regner A, Grivicich I, Pretto Schunemann D, Diel C, Kovaleski G, Brunetto De Farias C, Mondadori E, Almeida L, Braga Filho A, Schwartsmann G (2004) Radioresistance is associated to increased Hsp70 content in human glioblastoma cell lines. Int J Oncol 25:777–785
Carpentier AF, Chen L, Maltonti F, Delattre JY (1999) Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res 59:5429–5432
Dauphinee SM, Karsan A (2006) Lipopolysaccharide signaling in endothelial cells. Lab Invest 86:9–22
Egeter O, Mocikat R, Ghoreschi K, Dieckmann A, Rocken M (2000) Eradication of disseminated lymphomas with CpG-DNA activated T helper type 1 cells from nontransgenic mice. Cancer Res 60:1515–1520
Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, Giese T, Gires O, Endres S, Hartmann G (2003) Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res 63:6478–6487
Heckelsmiller K, Beck S, Rall K, Sipos B, Schlamp A, Tuma E, Rothenfusser S, Endres S, Hartmann G (2002) Combined dendritic cell- and CpG oligonucleotide-based immune therapy cures large murine tumors that resist chemotherapy. Eur J Immunol 32:3235–3245
Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H (2005) Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res 65:5009–5014
Lai P, Rabinowich H, Crowley-Nowick PA, Bell MC, Mantovani G, Whiteside TL (1996) Alterations in expression and function of signal-transducing proteins in tumor-associated T and natural killer cells in patients with ovarian carcinoma. Clin Cancer Res 2:161–173
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr (1997) A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature 388:394–397
Mozer-Lisewska I, Sluzewski W, Kaczmarek M, Jenek R, Szczepanski M, Figlerowicz M, Kowala-Piaskowska A, Zeromski J (2005) Tissue localization of Toll-like receptors in biopsy specimens of liver from children infected with hepatitis C virus. Scand J Immunol 62:407–412
Nanbu K, Konishi I, Mandai M, Kuroda H, Hamid AA, Komatsu T, Mori T (1998) Prognostic significance of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Cancer Detect Prev 22:549–555
Ohashi K, Burkart V, Flohe S, Kolb H (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164:558–561
Szczepanski M, Szyfter W, Jenek R, Wrobel M, Mozer-Lisewska I, Zeromski J (2006) Toll-like receptors 2, 3 and 4 (TLR-2, TLR-3 and TLR-4) are expressed in the microenvironment of human acquired cholesteatoma. Eur Arch Otorhinolaryngol 263:603–607
Triantafilou M, Manukyan M, Mackie A, Morath S, Hartung T, Heine H, Triantafilou K (2004) Lipoteichoic acid and toll-like receptor 2 internalization and targeting to the Golgi are lipid raft-dependent. J Biol Chem 279:40882–40889
Whiteside TL (2004) Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother 53:865–878
Whiteside TL (2006) Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol 16:3–15
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szczepański, M., Stelmachowska, M., Stryczyński, Ł. et al. Assessment of expression of toll-like receptors 2, 3 and 4 in laryngeal carcinoma. Eur Arch Otorhinolaryngol 264, 525–530 (2007). https://doi.org/10.1007/s00405-006-0215-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-006-0215-7