Abstract
Purpose
Hyperandrogenic intrauterine environment may lead to the development of metabolic disorders in offspring in their later life. In this study, we aimed to determine the impact of maternal hyperandrogenism (MHA) on metabolic syndrome (MetS) risk in female offspring in their later life.
Methods
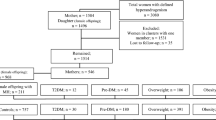
In this cohort study conducted in Tehran, Iran, female offspring with MHA (n = 323) and without MHA (controls) (n = 1125) were selected. Both groups of female offspring were followed from the baseline to the date of the incidence of events, censoring, or end of the study period, whichever came first. We used age-scaled unadjusted and adjusted Cox regression models to assess the hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between MHA and MetS in female offspring. The software package STATA was used for statistical analysis, and the significance level was set at P < 0.05.
Results
We observed a higher risk of MetS (unadjusted HR (95% CI), 1.36 (1.05–1.77)), (P = 0.02) and (adjusted HR (95% CI), 1.34 (1.00–1.80)), (P = 0.05, borderline)), in female offspring with MHA, compared to controls. The results were adjusted for the potential confounders including body mass index (BMI) at baseline, net changes of BMI, physical activity, education status, and birth weight.
Conclusion
Our results suggest that MHA increases the risk of developing MetS in female offspring in their later life. Screening of these female offspring for MetS may be recommended.



Similar content being viewed by others
References
Grundy SM (2005) Metabolic syndrome scientific statement by the american heart association and the national heart, lung, and blood institute. Arterioscler Thromb Vasc Biol 25:2243–2244. https://doi.org/10.1161/01.ATV.0000189155.75833.c7
Xita N, Tsatsoulis A (2010) Fetal origins of the metabolic syndrome. Ann N Y Acad Sci 1205:148–155. https://doi.org/10.1111/j.1749-6632.2010.05658.x
Recabarren SE, Petermann T, Maliqueo M, Lobos A, Rojas-García P (2006) Prenatal exposure to androgens as a factor of fetal programming. Rev Med Chil 134:101–108. https://doi.org/10.4067/s0034-98872006000100015
Hakim C, Padmanabhan V, Vyas AK (2017) Gestational hyperandrogenism in developmental programming. Endocrinology 158:199–212. https://doi.org/10.1210/en.2016-1801
Fowden AL, Forhead AJ (2004) Endocrine mechanisms of intrauterine programming. Reproduction 127:515–526. https://doi.org/10.1530/rep1.00033
Eisner JR, Dumesic DA, Kemnitz JW, Colman RJ, Abbott DH (2003) Increased adiposity in female rhesus monkeys exposed to androgen excess during early gestation. Obes Res 11:279–286. https://doi.org/10.1038/oby.2003.42
Silva AF, Abruzzese GA, Ferrer MJ, Heber MF, Ferreira SR, Cerrone GE, Motta AB (2022) Fetal programming by androgen excess impairs liver lipid content and PPARg expression in adult rats. J Dev Orig Health Dis 13:300–309. https://doi.org/10.1017/S2040174421000416
Chen X, Koivuaho E, Piltonen TT, Gissler M, Lavebratt C (2021) Association of maternal polycystic ovary syndrome or anovulatory infertility with obesity and diabetes in offspring: a population-based cohort study. Hum Reprod 36:2345–2357. https://doi.org/10.1093/humrep/deab112
Sir-Petermann T, Maliqueo M, Codner E, Echiburu B, Crisosto N, Perez V, Perez-Bravo F, Cassorla F (2007) Early metabolic derangements in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab 92:4637–4642. https://doi.org/10.1210/jc.2007-1036
Azizi F, Madjid M, Rahmani M, Emami H, Mirmiran P, Hadjipour R (2000) Tehran Lipid and Glucose Study (TLGS): rationale and design.
Azizi F (2018) Tehran lipid and glucose study: a national legacy. Int J Endocrinol Metab 16:e84774. https://doi.org/10.5812/ijem.84774
Tehrani FR, Rashidi H, Azizi F (2011) The prevalence of idiopathic hirsutism and polycystic ovary syndrome in the Tehran Lipid and Glucose Study. Reprod Biol Endocrinol 9:144. https://doi.org/10.1186/1477-7827-9-144
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF, Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen E, Society P (2009) The androgen excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91:456–488. https://doi.org/10.1016/j.fertnstert.2008.06.035
Reaspcw Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19:41–47. https://doi.org/10.1093/humrep/deh098
Dinh QQ, Sinclair R (2007) Female pattern hair loss: current treatment concepts. Clin Interv Aging 2:189–199
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC Jr (2009) Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120:1640–1645. https://doi.org/10.1161/Circulationaha.109.192644
Ncepepo Detection, Adults Tohbci, Third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). 2002: The Program.
Honaker J, King G, Blackwell M, Blackwell MM (2010) Package ‘Amelia’. Version.
Hsu CN, Hou CY, Hsu WH, Tain YL (2021) Early-Life origins of metabolic syndrome: mechanisms and preventive aspects. Int J Mol Sci 22:11872. https://doi.org/10.3390/ijms222111872
Bouchard L (2013) Epigenetics and fetal metabolic programming: a call for integrated research on larger cohorts. Diabetes 62:1026–1028. https://doi.org/10.2337/db12-1763
Gluckman P, Hanson M, Cooper C, Thornburg K (2008) Effect of in utero and earlylife conditions on adult health and disease. N Engl J Med 359:61–73. https://doi.org/10.1056/NEJMra0708473
Grayson DR, Guidotti A (2016) Merging data from genetic and epigenetic approaches to better understand autistic spectrum disorder. Epigenomics 8:85–104. https://doi.org/10.2217/epi.15.92
Santos MJ, Fernandes V, Marques O, Pereira ML (2016) Effect of maternal body mass index and weight gain in women with gestational diabetes on the incidence of large-for-gestational-age infants. Diabetes Metab 42:471–474. https://doi.org/10.1016/j.diabet.2016.06.008
Wang D, Xu S, Chen H, Zhong L, Wang Z (2015) The associations between triglyceride to high-density lipoprotein cholesterol ratios and the risks of gestational diabetes mellitus and large-for-gestational-age infant. Clin Endocrinol 83:490–497. https://doi.org/10.1111/cen
Mossa F, Latham KE, Ireland JJ, Veiga-Lopez A (2019) Undernutrition and hyperandrogenism during pregnancy: role in programming of cardiovascular disease and infertility. Mol Reprod Dev 86:1255–1264. https://doi.org/10.1002/mrd.23239
Puttabyatappa M, Sargis RM, Padmanabhan V (2020) Developmental programming of insulin resistance: are androgens the culprits? J Endocrinol 245:R23–R48. https://doi.org/10.1530/JOE-20-0044
Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE (2002) Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod 17:2573–2579. https://doi.org/10.1093/humrep/17.10.2573
Dumesic DA, Goodarzi MO, Chazenbalk GD, Abbott DH (2014) Intrauterine environment and polycystic ovary syndrome. Semin Reprod Med 32:159–165. https://doi.org/10.1055/s-0034-1371087
Padmanabhan V, Manikkam M, Recabarren S, Foster D (2006) Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol 246:165–174. https://doi.org/10.1016/j.mce.2005.11.016
Cardoso RC, Veiga-Lopez A, Moeller J, Beckett E, Pease A, Keller E, Madrigal V, Chazenbalk G, Dumesic D, Padmanabhan V (2016) Developmental programming: impact of gestational steroid and metabolic milieus on adiposity and insulin sensitivity in prenatal testosterone-treated female sheep. Endocrinology 157:522–535. https://doi.org/10.1210/en.2015-1565
Carrasco A, Recabarren MP, Rojas-Garcia PP, Gutierrez M, Morales K, Sir-Petermann T, Recabarren SE (2020) Prenatal testosterone exposure disrupts insulin secretion and promotes insulin resistance. Sci Rep 10:404. https://doi.org/10.1038/s41598-019-57197-x
Sherman SB, Sarsour N, Salehi M, Schroering A, Mell B, Joe B, Hill JW (2018) Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes 9:400–421. https://doi.org/10.1080/19490976.2018.1441664
Yildiz BO, Yarali H, Oguz H, Bayraktar M (2003) Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J Clin Endocrinol Metab 88:2031–2036. https://doi.org/10.1210/jc.2002-021499
Eisner JR, Dumesic DA, Kemnitz JW, Abbott DH (2000) Timing of prenatal androgen excess determines differential impairment in insulin secretion and action in adult female rhesus monkeys. J Clin Endocrinol Metab 85:1206–1210. https://doi.org/10.1210/jcem.85.3.6453
Recabarren SE, Padmanabhan V, Codner E, Lobos A, Duran C, Vidal M, Foster DL, Sir-Petermann T (2005) Postnatal developmental consequences of altered insulin sensitivity in female sheep treated prenatally with testosterone. Am J Physiol Endocrinol Metab 289:E801-806. https://doi.org/10.1152/ajpendo.00107.2005
Noroozzadeh M, Ramezani Tehrani F, Sedaghat K, Godini A, Azizi F (2015) The impact of prenatal exposure to a single dose of testosterone on insulin resistance, glucose tolerance and lipid profile of female rat’s offspring in adulthood. J Endocrinol Invest 38:489–495. https://doi.org/10.1007/s40618-014-0198-y
Daan NM, Koster MP, Steegers-Theunissen RP, Eijkemans MJ, Fauser B (2017) Endocrine and cardiometabolic cord blood characteristics of offspring born to mothers with and without polycystic ovary syndrome. Fertil Steril 107:261–268. https://doi.org/10.1016/j.fertnstert.2016.09.042
Mehrabian F, Khani B (2014) Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. J Res Med Sci 17:207–211
Veiga-Lopez A, Moeller J, Patel D, Ye W, Pease A, Kinns J, Padmanabhan V (2013) Developmental programming: impact of prenatal testosterone excess on insulin sensitivity, adiposity, and free fatty acid profile in postpubertal female sheep. Endocrinology 154:1731–1742. https://doi.org/10.1210/en.2012-2145
Noroozzadeh M, Rahmati M, Behboudi-Gandevani S, Ramezani Tehrani F (2022) Maternal hyperandrogenism is associated with a higher risk of type 2 diabetes mellitus and overweight in adolescent and adult female offspring: a long-term population-based follow-up study. J Endocrinol Invest 45:963–972. https://doi.org/10.1007/s40618-021-01721-2
Li J, Daly E, Campioli E, Wabitsch M, Papadopoulos V (2014) De novo synthesis of steroids and oxysterols in adipocytes. J Biol Chem 289:747–764. https://doi.org/10.1074/jbc.M113.534172
Kandel DB, Udry JR (1999) Prenatal effects of maternal smoking on daughters’ smoking: nicotine or testosterone exposure? Am J Public Health 89:1377–1383. https://doi.org/10.2105/ajph.89.9.1377
Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G (2006) Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur J Obstet Gynecol Reprod Biol 126:16–19. https://doi.org/10.1016/j.ejogrb.2005.07.007
Palioura E, Diamanti-Kandarakis E (2015) Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev Endocr Metab Disord 16:365–371. https://doi.org/10.1007/s11154-016-9326-7
Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V (2004) Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 145:790–798. https://doi.org/10.1210/en.2003-0478
Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C (2011) Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol 9:1–12. https://doi.org/10.1186/1477-7827-9-110
Grigore D, Ojeda NB, Alexander BT (2008) Sex differences in the fetal programming of hypertension. Gend Med. https://doi.org/10.1016/j.genm.2008.03.012
Nada SE, Thompson RC, Padmanabhan V (2010) Developmental programming: differential effects of prenatal testosterone excess on insulin target tissues. Endocrinology 151:5165–5173. https://doi.org/10.1210/en.2010-0666
Sathishkumar K, Elkins R, Yallampalli U, Balakrishnan M, Yallampalli C (2011) Fetal programming of adult hypertension in female rat offspring exposed to androgens in utero. Early Hum Dev 87:407–414. https://doi.org/10.1016/j.earlhumdev.2011.03.001
Filippou P, Homburg R (2017) Is foetal hyperexposure to androgens a cause of PCOS? Hum Reprod Update 23:421–432. https://doi.org/10.1093/humupd/dmx013
Behboudi-Gandevani S, Ramezani Tehrani F, Hosseinpanah F, Khalili D, Cheraghi L, Kazemijaliseh H, Azizi F (2018) Cardiometabolic risks in polycystic ovary syndrome: long-term population-based follow-up study. Fertil Steril 110(7):1377
Fernández-Rhodes L, Young KL, Lilly AG, Raffield LM, Highland HM, Wojcik GL, Agler C, Love S-AM, Okello S, Petty LE (2020) Importance of genetic studies of cardiometabolic disease in diverse populations. Circ Res 126:1816–1840. https://doi.org/10.1161/CIRCRESAHA.120.315893
Huang G, Cherkerzian S, Loucks EB, Buka SL, Handa RJ, Lasley BL, Bhasin S, Goldstein JM (2018) Sex differences in the prenatal programming of adult metabolic syndrome by maternal androgens. J Clin Endocrinol Metab 103(11):3945–3953. https://doi.org/10.1210/jc.2018-01243
Zhou Y, Gong M, Lu Y, Chen J, Ju R (2021) Prenatal androgen excess impairs beta-cell function by decreased sirtuin 3 expression. J Endocrinol 251:69–81. https://doi.org/10.1530/JOE-21-0129
Roland AV, Nunemaker CS, Keller SR, Moenter SM (2010) Prenatal androgen exposure programs metabolic dysfunction in female mice. J Endocrinol 207:213–223. https://doi.org/10.1677/JOE-10-0217
Acknowledgements
We thank the laboratory staff for helping us to measure all blood parameters (hormones and biochemical parameters). This work was financially supported by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Funding
This work was financially supported by the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran (Grand number 43002403)
Author information
Authors and Affiliations
Contributions
MN, FRT, and FA contributed to the study conception and design. Material preparation, data collection and analysis were performed by MN, MR, MF-A, and MSGN. The first draft of the manuscript was written by MN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that there are no conflicts of interest.
Ethics approval
The ethics review board of the Research Institute for Endocrine Sciences approved the study proposal.
Consent to participate
Written informed consent was signed by all participants, after a full explanation of the purpose of the study to them. Written consent was obtained from their parents, if they were under 18 years old.
Consent to publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Noroozzadeh, M., Rahmati, M., Farhadi-Azar, M. et al. Maternal androgen excess increases the risk of metabolic syndrome in female offspring in their later life: A long-term population-based follow-up study. Arch Gynecol Obstet 308, 1555–1566 (2023). https://doi.org/10.1007/s00404-023-07132-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-023-07132-3