Abstract
Purpose
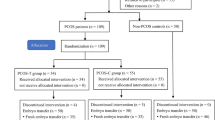
The aim of the present study was to evaluate the effect of myo-Inositol administration on oocyte quality, fertilization rate and embryo quality in patients with PCOS during assisted reproductive technology (ART) cycles.
Methods
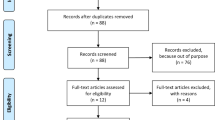
Fifty infertile PCOS patients were randomly designated in two groups. In the study group, patients received daily doses of 4 g myo-Inositol combined with 400 mg folic acid and in the control group patients received only 400 mg folic acid from 1 month before starting the antagonist cycle until the day of ovum pick up. Oocyte and embryo qualities were assessed according to European Society of Human Reproduction and Embryology (ESHRE) guidelines. The gene expression of PGK1, RGS2 and CDC42 as a factor of oocyte quality in granulosa cells was analyzed using real-time RT-PCR. Levels of total antioxidant capacity (TAC) and reactive oxygen species (ROS) were evaluated by chemiluminescence assay in follicular fluid.
Results
The percentage of metaphase II oocyte, fertilization rate and embryo quality significantly improved in the study group (p < 0.05), but the number of retrieved oocytes and follicle count were not statistically different between groups. Furthermore, the gene expression of PGK1, RGS2 and CDC42 was significantly higher in the study group (p < 0.05) but no differences were found between two groups in terms of TAC and ROS levels.
Conclusions
The present study findings suggest that myo-Inositol alters the gene expression in granulosa cells and improves oocyte and embryo quality among PCOS patients undergoing ART.


Similar content being viewed by others
References
El-Berry S, Razik MA (2010) Nitric oxide donors increases pregnancy rate in clomiphene citrate treated polycystic ovary infertile patients. Middle East Fertil Soc J 15(2):106–109
Amjadi F et al (2018) Distinct changes in the proteome profile of endometrial tissues in polycystic ovary syndrome compared with healthy fertile women. RBM Online 37(2):184–200
Chen S, Song J (2008) Oocyte quality and embryo quality of infertile women with polycystic ovarian syndrome. Fertil Steril 90:S132
Teede HJ et al (2018) Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod 33(9):1602–1618
Salehpour S et al (2012) N-Acetylcysteine as an adjuvant to clomiphene citrate for successful induction of ovulation in infertile patients with polycystic ovary syndrome. J Obstet Gynaecol Res 38(9):1182–1186
Salehi E et al (2017) Apoptotic biomarkers in cumulus cells in relation to embryo quality in polycystic ovary syndrome. Arch Gynecol Obstet 296(6):1219–1227
Nordio M, Proietti E (2012) The combined therapy with myo-inositol and D-chiro-inositol reduces the risk of metabolic disease in PCOS overweight patients compared to myo-inositol supplementation alone. Eur Rev Med Pharmacol Sci 16(5):575–581
Phillippy BQ, Graf E (1997) Antioxidant functions of inositol 1,2,3-trisphosphate and inositol 1,2,3,6-tetrakisphosphate. Free Radic Biol Med 22(6):939–946
Pundir J et al (2018) Inositol treatment of anovulation in women with polycystic ovary syndrome: a meta-analysis of randomised trials. BJOG 125(3):299–308
Croze ML, Soulage CO (2013) Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 95(10):1811–1827
Foster SR et al (2017) Effects of combined inositol hexakisphosphate and inositol supplement on antioxidant activity and metabolic enzymes in the liver of streptozotocin-induced type 2 diabetic rats. Chem Biol Interact 275:108–115
Ciotta L et al (2011) Effects of myo-inositol supplementation on oocyte's quality in PCOS patients: a double blind trial. Eur Rev Med Pharmacol Sci 15(5):509–514
Vartanyan EV et al (2017) Improvement in quality of oocytes in polycystic ovarian syndrome in programs of in vitro fertilization. Gynecol Endocrinol 33(sup1):8–11
Uyar A, Torrealday S, Seli E (2013) Cumulus and granulosa cell markers of oocyte and embryo quality. Fertil Steril 99(4):979–997
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Gu BX et al (2016) Abnormal expression of TLRs may play a role in lower embryo quality of women with polycystic ovary syndrome. Syst Biol Reprod Med 62(5):353–358
Benner A (1999) Sample size tables for clinical studies. (2nd edn). In: David Machin, Michael JC, Peter MF, Alain PY, Pinol, Blackwell Science Ltd., Oxford, 1997. No. of pages: x+315. Price: £45. ISBN 0-86542-870-0. Stat Med 18(4):494–495
Rago R et al (2015) Effect of myo-inositol and alpha-lipoic acid on oocyte quality in polycystic ovary syndrome non-obese women undergoing in vitro fertilization: a pilot study. J Biol Regul Homeost Agents 29(4):913–923
Amjadi F et al (2015) Apolipoprotein A1 as a novel anti-implantation biomarker in polycystic ovary syndrome: a case-control study. J Res Med Sci 20(11):1039
Pacchiarotti A et al (2016) Effect of myo-inositol and melatonin versus myo-inositol, in a randomized controlled trial, for improving in vitro fertilization of patients with polycystic ovarian syndrome. Gynecol Endocrinol 32(1):69–73
Ocal P et al (2012) Recurrent implantation failure is more frequently seen in female patients with poor prognosis. Int J Fertil Steril 6(2):71–78
Papaleo E, et al (2009) Myo-inositol may improve oocyte quality in intracytoplasmic sperm injection cycles. A prospective, controlled, randomized trial. Fertil Steril 91(5):1750–1754
Emekci Ozay O et al (2017) Myo-inositol administration positively effects ovulation induction and intrauterine insemination in patients with polycystic ovary syndrome: a prospective, controlled, randomized trial. Gynecol Endocrinol 33(7):524–528
Unfer V et al (2011) Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol 27(11):857–861
Mendoza N et al (2017) Inositol supplementation in women with polycystic ovary syndrome undergoing intracytoplasmic sperm injection: a systematic review and meta-analysis of randomized controlled trials. Reprod Biomed Online 35(5):529–535
Ajduk A, Malagocki A, Maleszewski M (2008) Cytoplasmic maturation of mammalian oocytes: development of a mechanism responsible for sperm-induced Ca2+ oscillations. Reprod Biol 8(1):3–22
Hammes SR (2004) Steroids and oocyte maturation–a new look at an old story. Mol Endocrinol 18(4):769–775
Wang Q, Moley KH (2010) Maternal diabetes and oocyte quality. Mitochondrion 10(5):403–410
Nelson VL et al (1999) Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol 13(6):946–957
Unfer V et al (2017) Myo-inositol effects in women with PCOS: a meta-analysis of randomized controlled trials. Endocr Connect 6(8):647–658
Zeng L, Yang K (2018) Effectiveness of myoinositol for polycystic ovary syndrome: a systematic review and meta-analysis. Endocrine 59(1):30–38
Bevilacqua A et al (2018) Myo-inositol and D-chiro-inositol (40:1) reverse histological and functional features of polycystic ovary syndrome in a mouse model. J Cell Physiol 234(6):9387–9398
Ducibella T, Schultz RM, Ozil JP (2006) Role of calcium signals in early development. Semin Cell Dev Biol 17(2):324–332
Condorelli RA et al (2011) Effects of myoinositol on sperm mitochondrial function in-vitro. Eur Rev Med Pharmacol Sci 15(2):129–134
Showell MG et al (2018) Inositol for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev 12:CD012378
Karuputhula NB et al (2013) Oxidative status in granulosa cells of infertile women undergoing IVF. Syst Biol Reprod Med 59(2):91–98
Bernhardt ML et al (2015) Regulator of G-protein signaling 2 (RGS2) suppresses premature calcium release in mouse eggs. Development 142(15):2633–2640
Hamel M et al (2010) Genomic assessment of follicular marker genes as pregnancy predictors for human IVF. Mol Hum Reprod 16(2):87–96
Gu L et al (2015) Metabolic control of oocyte development: linking maternal nutrition and reproductive outcomes. Cell Mol Life Sci 72(2):251–271
Mikaeili S et al (2016) Altered FoxO3 expression and apoptosis in granulosa cells of women with polycystic ovary syndrome. Arch Gynecol Obstet 294(1):185–192
Varras M et al (2012) Expression of antiapoptosis gene survivin in luteinized ovarian granulosa cells of women undergoing IVF or ICSI and embryo transfer: clinical correlations. Reprod Biol Endocrinol 10:74
Patel SS, Carr BR (2008) Oocyte quality in adult polycystic ovary syndrome. Semin Reprod Med 26(2):196–203
Tu S, Cerione RA (2001) Cdc42 Is a Substrate for Caspases and Influences Fas-induced Apoptosis. J Biol Chem 276(22):19656–19663
Acknowledgements
The authors would like to thank Professor Felice Petraglia (University of Florence, Italy) for his input and guidance in revising our manuscript. This study was financed by Iran University of Medical Science (Grant no. 26493).
Author information
Authors and Affiliations
Contributions
A.A.S: substantial contributions to the conception or design, interpretation of data and final approval of the version to be published; A.T: acquisition of data and collecting samples; H.N: acquisition of data; A.A: statistical analysis and interpretation of data; K.S: drafting and english editing of the article; M.M.A: acquisition of data; M.T: acquisition of data; M.A: revision the article critically for the important intellectual contents; F.A: substantial contributions to the conception or design, drafting the article and final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest with the subject matter of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akbari Sene, A., Tabatabaie, A., Nikniaz, H. et al. The myo-inositol effect on the oocyte quality and fertilization rate among women with polycystic ovary syndrome undergoing assisted reproductive technology cycles: a randomized clinical trial. Arch Gynecol Obstet 299, 1701–1707 (2019). https://doi.org/10.1007/s00404-019-05111-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05111-1