Abstract
Introduction
Lynch syndrome is known by healthcare providers mainly for patients with colorectal cancer. Awareness of other associated tumors, such as endometrial or ovarian cancer, is low. This study aimed to analyze the prevalence of Lynch syndrome in unselected patients with endometrial or ovarian cancer. In addition, the willingness of patients and family members to undergo germline mutation testing was investigated.
Methods
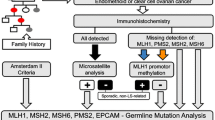
The medical records of all patients diagnosed with endometrial or ovarian cancer at the Department of Gynecology and Obsterics, University Hospital Dresden, between 1998 and 2012, were screened for a family history of HNPCC-associated cancer. Telephone interviews were used to screen, inform, and enroll patients in this genetic analysis study. Molecular analysis was performed by prescreening of tumor tissue, followed by germline mutation analysis.
Results
Two hundred and eighty-three patients were diagnosed with endometrial cancer, 291 with ovarian cancer, and 14 with both. A positive family history for tumors associated with Lynch syndrome was documented for 61 patients. Two pathogenic mutations in the genes MLH1 and MSH2 in nine genetic analyses had already been detected before. After genetic counseling, only 10 of 31 index patients (32.3 %) consented for mutation analysis. However, no additional pathogenic heterozygous mutations were found.
Conclusion
In this retrospective cohort study in unselected patients with endometrial or ovarian cancer, only a small number of patients with suspected Lynch syndrome could be identified. Of those, acceptance of germline analyses was moderate, only. As a result, the rate of identified pathogenic germline mutations was lower than expected. Therefore, we are convinced that more information on cancer risks, options for predictive molecular testing and preventive procedures, needs to be provided to patients and gynecologists.
Similar content being viewed by others
References
Lynch HT, de la Chapelle A (2003) Hereditary colorectal cancer. N Engl J Med 348(10):919–932. doi:10.1056/NEJMra012242
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S (2004) Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 96(4):261–268
Moller P, Seppala T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, Lindblom A, Macrae F, Blanco I, Sijmons R, Jeffries J, Vasen H, Burn J, Nakken S, Hovig E, Rodland EA, Tharmaratnam K, de Vos Tot Nederveen Cappel WH, Hill J, Wijnen J, Green K, Lalloo F, Sunde L, Mints M, Bertario L, Pineda M, Navarro M, Morak M, Renkonen-Sinisalo L, Frayling IM, Plazzer JP, Pylvanainen K, Sampson JR, Capella G, Mecklin JP, Moslein G (2015) Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. doi:10.1136/gutjnl-2015-309675
Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, de la Chapelle A, Peltomaki P, Mecklin JP, Jarvinen HJ (1999) Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 81(2):214–218
Baglietto L, Lindor NM, Dowty JG, White DM, Wagner A, Gomez Garcia EB, Vriends AH, Cartwright NR, Barnetson RA, Farrington SM, Tenesa A, Hampel H, Buchanan D, Arnold S, Young J, Walsh MD, Jass J, Macrae F, Antill Y, Winship IM, Giles GG, Goldblatt J, Parry S, Suthers G, Leggett B, Butz M, Aronson M, Poynter JN, Baron JA, Le Marchand L, Haile R, Gallinger S, Hopper JL, Potter J, de la Chapelle A, Vasen HF, Dunlop MG, Thibodeau SN, Jenkins MA (2010) Risks of Lynch syndrome cancers for MSH6 mutation carriers. J Natl Cancer Inst 102(3):193–201. doi:10.1093/jnci/djp473
Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, Guimbaud R, Buecher B, Bignon YJ, Caron O, Colas C, Nogues C, Lejeune-Dumoulin S, Olivier-Faivre L, Polycarpe-Osaer F, Nguyen TD, Desseigne F, Saurin JC, Berthet P, Leroux D, Duffour J, Manouvrier S, Frebourg T, Sobol H, Lasset C, Bonaiti-Pellie C (2011) Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA J Am Med Assoc 305(22):2304–2310. doi:10.1001/jama.2011.743
Dunlop MG, Farrington SM, Carothers AD, Wyllie AH, Sharp L, Burn J, Liu B, Kinzler KW, Vogelstein B (1997) Cancer risk associated with germline DNA mismatch repair gene mutations. Hum Mol Genet 6(1):105–110
Walsh CS, Blum A, Walts A, Alsabeh R, Tran H, Koeffler HP, Karlan BY (2010) Lynch syndrome among gynecologic oncology patients meeting Bethesda guidelines for screening. Gynecol Oncol 116(3):516–521. doi:10.1016/j.ygyno.2009.11.021
Hampel H, Frankel W, Panescu J, Lockman J, Sotamaa K, Fix D, Comeras I, La Jeunesse J, Nakagawa H, Westman JA, Prior TW, Clendenning M, Penzone P, Lombardi J, Dunn P, Cohn DE, Copeland L, Eaton L, Fowler J, Lewandowski G, Vaccarello L, Bell J, Reid G, de la Chapelle A (2006) Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 66(15):7810–7817. doi:10.1158/0008-5472.can-06-1114
Berends MJ, Wu Y, Sijmons RH, van der Sluis T, Ek WB, Ligtenberg MJ, Arts NJ, ten Hoor KA, Kleibeuker JH, de Vries EG, Mourits MJ, Hollema H, Buys CH, Hofstra RM, van der Zee AG (2003) Toward new strategies to select young endometrial cancer patients for mismatch repair gene mutation analysis. J Clin Oncol 21(23):4364–4370. doi:10.1200/jco.2003.04.094
Lu KH, Schorge JO, Rodabaugh KJ, Daniels MS, Sun CC, Soliman PT, White KG, Luthra R, Gershenson DM, Broaddus RR (2007) Prospective determination of prevalence of lynch syndrome in young women with endometrial cancer. J Clin Oncol 25(33):5158–5164. doi:10.1200/jco.2007.10.8597
Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, Lynch H, Perucho M, Smyrk T, Sobin L, Srivastava S (1997) A National Cancer Institute workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst 89(23):1758–1762
Plaschke J, Kruger S, Pistorius S, Theissig F, Saeger HD, Schackert HK (2002) Involvement of hMSH6 in the development of hereditary and sporadic colorectal cancer revealed by immunostaining is based on germline mutations, but rarely on somatic inactivation. Int J Cancer 97(5):643–648
Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, Ramirez N, Pritchard CC, Hampel H, Chassen AS, Simmons LV, Schmidt AP, Gao F, Brinton LA, Backes F, Landrum LM, Geller MA, DiSilvestro PA, Pearl ML, Lele SB, Powell MA, Zaino RJ, Mutch D (2015) Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG oncology and gynecologic oncology group study. J Clin Oncol 33(36):4301–4308. doi:10.1200/jco.2015.63.9518
Egoavil C, Alenda C, Castillejo A, Paya A, Peiro G, Sanchez-Heras AB, Castillejo MI, Rojas E, Barbera VM, Ciguenza S, Lopez JA, Pinero O, Roman MJ, Martinez-Escoriza JC, Guarinos C, Perez-Carbonell L, Aranda FI, Soto JL (2013) Prevalence of Lynch syndrome among patients with newly diagnosed endometrial cancers. PLoS One 8(11):e79737. doi:10.1371/journal.pone.0079737
Hampel H, Panescu J, Lockman J, Sotamaa K, Fix D, Comeras I, LaJeunesse J, Nakagawa H, Westman JA, Prior TW, Clendenning M, de la Chapelle A, Frankel W, Penzone P, Cohn DE, Copeland L, Eaton L, Fowler J, Lombardi J, Dunn P, Bell J, Reid G, Lewandowski G, Vaccarello L (2007) Comment on: screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 67(19):9603. doi:10.1158/0008-5472.can-07-2308
Moline J, Mahdi H, Yang B, Biscotti C, Roma AA, Heald B, Rose PG, Michener C, Eng C (2013) Implementation of tumor testing for lynch syndrome in endometrial cancers at a large academic medical center. Gynecol Oncol 130(1):121–126. doi:10.1016/j.ygyno.2013.04.022
Leenen CH, van Lier MG, van Doorn HC, van Leerdam ME, Kooi SG, de Waard J, Hoedemaeker RF, van den Ouweland AM, Hulspas SM, Dubbink HJ, Kuipers EJ, Wagner A, Dinjens WN, Steyerberg EW (2012) Prospective evaluation of molecular screening for Lynch syndrome in patients with endometrial cancer ≤70 years. Gynecol Oncol 125(2):414–420. doi:10.1016/j.ygyno.2012.01.049
Malander S, Rambech E, Kristoffersson U, Halvarsson B, Ridderheim M, Borg A, Nilbert M (2006) The contribution of the hereditary nonpolyposis colorectal cancer syndrome to the development of ovarian cancer. Gynecol Oncol 101(2):238–243. doi:10.1016/j.ygyno.2005.10.029
Pal T, Akbari MR, Sun P, Lee JH, Fulp J, Thompson Z, Coppola D, Nicosia S, Sellers TA, McLaughlin J, Risch HA, Rosen B, Shaw P, Schildkraut J, Narod SA (2012) Frequency of mutations in mismatch repair genes in a population-based study of women with ovarian cancer. Br J Cancer 107(10):1783–1790. doi:10.1038/bjc.2012.452
Steinke V, Holzapfel S, Loeffler M, Holinski-Feder E, Morak M, Schackert HK, Gorgens H, Pox C, Royer-Pokora B, von Knebel-Doeberitz M, Buttner R, Propping P, Engel C (2014) Evaluating the performance of clinical criteria for predicting mismatch repair gene mutations in Lynch syndrome: a comprehensive analysis of 3,671 families. Int J Cancer 135(1):69–77. doi:10.1002/ijc.28650
Batte BA, Bruegl AS, Daniels MS, Ring KL, Dempsey KM, Djordjevic B, Luthra R, Fellman BM, Lu KH, Broaddus RR (2014) Consequences of universal MSI/IHC in screening endometrial cancer patients for Lynch syndrome. Gynecol Oncol 134(2):319–325. doi:10.1016/j.ygyno.2014.06.009
Bach K, Preyer J, Jensen A, Epplen JT, Kunstmann E (2006) Gynecological outpatient management in HNPCC. Zentralbl Gynakol 128(4):207–212. doi:10.1055/s-2006-933430
Ketabi Z, Mosgaard BJ, Gerdes AM, Ladelund S, Bernstein IT (2012) Awareness of endometrial cancer risk and compliance with screening in hereditary nonpolyposis colorectal cancer. Obstet Gynecol 120(5):1005–1012. doi:10.1097/AOG.0b013e31826ba2aa
Acknowledgments
We would like to thank the German Consortium for Hereditary Non-Polyposis Colorectal Cancer (supported by the German Cancer Aid) for providing infrastructure and guidelines for interdisciplinary care. We would like to thank Prof. Dr. Hans K. Schackert and Dr. Heike Schackert for inspiration to do the work, molecular genetic analysis and proof-reading the manuscript. We thank Michael Hanna, Ph.D., for proof-reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was provided for this study.
Conflict of interest
All authors declare no potential conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study has been approved by the institutional review board on September 25th 2012, No EK 183062012.
Informed consent
Informed consent was obtained from all individual participants included in the study before molecular genetic testing.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kast, K., Dobberschütz, C., Sadowski, C.E. et al. Prevalence of Lynch syndrome in unselected patients with endometrial or ovarian cancer. Arch Gynecol Obstet 294, 1299–1303 (2016). https://doi.org/10.1007/s00404-016-4180-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4180-0