Abstract
Purpose
This preliminary study aimed at investigating the feasibility and effective of multi-scale hyperspectral imaging in detecting cervical neoplasia at both tissue and cellular levels.
Methods
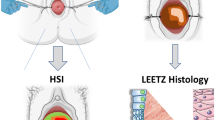
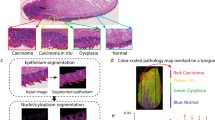
In this paper, we describe a noninvasive diagnosis method with a hyperspectral imager for detection and location of cervical intraepithelial neoplasia (CIN) at multiple scales. At the macroscopic level, the hyperspectral imager was applied to capture the reflectance images of the entire cervix in vivo at a series of wavelengths. At the microscopic level, the hyperspectral imager was coupled with a microscope to collect the transmittance images of the pathological slide. The collected image data were calibrated. A wide-gap second derivative analysis was applied to differentiate CIN from other types of tissue.
Results
At both macroscopic and microscopic levels, hyperspectral imaging analysis results were consistent with those of histopathological analysis, indicating the technical feasibility of multi-scale hyperspectral imaging for cervical neoplasia detection with accuracy and efficacy.
Conclusion
We propose a multi-scale hyperspectral imaging method for noninvasive detection of cervical neoplasia. Comparison of the imaging results with those of gold standard histologic measurements demonstrates that the hyperspectral diagnostic imaging system can distinguish CIN at both tissue and cellular levels.






Similar content being viewed by others
References
http://www.chinacdc.cn/mtdx/mxfcrxjbxx/201405/t20140513_96910.htm. Accessed 2014
Walboomers JMM, Jacobs MV, Manos MM et al (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189:12–19. doi:10.1002/(sici)1096-9896(199909)189:1<12:aid-path431>3.0.co;2-f
Barreto CL, Martins DBG et al (2013) Detection of Human Papillomavirus in biopsies of patients with cervical cancer. Arch Gynecol Obstet 288(3):643–648. doi:10.1007/s00404-013-3042-2
Bosch FX, Manos MM, MunB G et al (1995) Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst 87:796bste
Orfanoudaki IM, Kappou D, Sifakis S (2011) Recent advances in optical imaging for cervical cancer detection. Arch Gynecol Obstet 284(5):1197–1208. doi:10.1007/s00404-011-2009-4
Akbari H, Uto K, Kosugi Y et al (2011) Cancer detection using infrared hyperspectral imaging. Cancer Sci 102:852–857. doi:10.1111/j.1349-7006.2011.01849.x
Roblyer D, Kurachi C, Gillenwater AM et al (2009) In vivo fluorescence hyperspectral imaging of oral neoplasia. Proc SPIE 7169, 71690J-1–10
Gustafsson UP, McLaughlin E, Jacobsen E et al (2003) In-vivo fluorescence and reflectance imaging of human cervical tissue. Proc SPIE 5031:521–530
Ferris DG, Lawhead RA, Dickman ED et al (2001) Multimodal hyperspectral imaging for the noninvasive diagnosis of cervical neoplasia. J Lower Genital Tract Dis 5:65–72. doi:10.1046/j.1526-0976.2001.005002065.x
Zeng H, Zhao J, Short M et al (2008) Raman spectroscopy for in vivo tissue analysis and diagnosis, from instrument development to clinical applications. J Innov Opt Health Sci 1(1):98–106. doi:10.1142/S1793545808000054
Singh SP, Deshmukh A, Chaturvedi P et al (2012) In vivo Raman spectroscopic identification of premalignant lesions in oral buccal mucosa. J Biomed Opt 17(10):1050021–1050029. doi:10.1117/1.JBO.17.10.105002
Rubina S, Vidyasagar MS, Murali KC (2013) Raman spectroscopic study on prediction of treatment response in cervical cancers. J Innov Opt Health Sci 6(2):1350014. doi:10.1142/S1793545813500144
Pinto F, Mielewczik M, Liebisch F et al (2013) Non-invasive measurement of frog skin reflectivity in high spatial resolution using a dual hyperspectral approach. PLoS One 8(9):e73234. doi:10.1371/journal.pone.0073234
Mirabal YN, Chang SK, Atkinson EN et al (2002) Reflectance spectroscopy for in vivo detection of cervical precancer. J Biomed Opt 7(4):587–594. doi:10.1117/1.1502675
Mourant JR, Bocklage TJ, Powers TM et al (2007) In vivo light scattering measurements for detection of precancerous conditions of the cervix. Gynecol Oncol 105:439–445. doi:10.1016/j.ygyno.2007.01.001
Wang A, Nammalavar V, Drezek R (2007) Targeting spectral signatures of progressively dysplastic stratified epithelia using angularly variable fiber geometry in reflectance Monte Carlo simulations. J Biomed Opt 12(4):044012. doi:10.1117/1.2769328
Tabrizi SH, Aghamiri SMR, Farzaneh F et al (2014) The use of optical spectroscopy for in vivo detection of cervical pre-cancer. Lasers Med Sci 29:831–845
Roblyer D, Park SY, Richards-Kortum R et al (2007) Objective screening for cervical cancer in developing nations: lessons from Nigeria. Gynecol Oncol 107:S94–S97. doi:10.1016/j.ygyno.2007.07.042
Karpińska J (2012) Derivative spectrophotometry-recent applications and directions of developments. Talanta 64(4):801–822
Wachman ES, Geyer SJ, Recht JM et al (2014) Simultaneous imaging of cellular morphology and multiple biomarkers using an acousto-optic tunable filter-based bright field microscope. J Biomed Opt 19(5):0560061–05600614. doi:10.1117/1.JBO.19.5.056006
Stratis DN, Eland KL, Carter JC et al (2001) Comparison of acousto-optic and liquid crystal tunable filters for laser-induced breakdown spectroscopy. Appl Spectrosc 55(8):999–1004
Becker BL, Lusch DP, Qi J (2005) Identifying optimal spectral bands from in situ measurements of Great Lakes coastal wetlands using second-derivative analysis. Remote Sens Environ 97:238–248. doi:10.1016/j.rse.2005.04.020
Fabrizii M, Moinfar F, Jelinek HF et al (2014) Fractal analysis of cervical intraepithelial neoplasia. PLoS One 9(10):e108457. doi:10.1371/journal.pone.0108457
Nordstrom RJ, Burke L, Niloff JM et al (2001) Identification of cervical intraepithelial neoplasia (CIN) using UV-excited fluorescence and diffuse-reflectance tissue spectroscopy. Lasers Surg Med 29:118–127. doi:10.1002/lsm.1097
Chang SK, Mirabal YN, Atkinson EN et al (2005) Combined reflectance and fluorescence spectroscopy for in vivo detection of cervical pre-cancer. J Biomed Opt 10(2):024031. doi:10.1117/1.1899686
Thekkek N, Richards-Kortum R (2008) Optical imaging for cervical cancer detection: solutions for a continuing global problem. Nat Rev Cancer 8(9):725–731. doi:10.1038/nrc2462
Morimoto S, McClure WF, Stanfield DL (2001) Hand-held NIR spectrometry: Part I: an instrument based upon gap-second derivative theory. Appl Spectrosc 55(2):182–189
Balas C (2001) A novel optical imaging method for the early detection, quantitative grading, and mapping of cancerous and precancerous lesions of cervix. Biomed Eng IEEE Trans 48(1):96–104. doi:10.1109/10.900259
Collier T, Follen M, Malpica A et al (2005) Sources of scattering in cervical tissue: determination of the scattering coefficient by confocal microscopy. Appl Opt 44(11):2072–2081. doi:10.1364/AO.44.002072
Acknowledgments
This project was partially supported by Natural Science Foundation of China (Nos. 81271527 and 81327803) and the Fundamental Research Funds for the Central Universities (WK2090090013). The authors are grateful for the help of the traceable reflectance standard provided by Dr. David Allen at National Institute of Standards and Technology (NIST).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Wang, C., Zheng, W., Bu, Y. et al. Multi-scale hyperspectral imaging of cervical neoplasia. Arch Gynecol Obstet 293, 1309–1317 (2016). https://doi.org/10.1007/s00404-015-3906-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3906-8