Abstract
Background
Hypoxia-inducible factor-1 (HIF-1) is an essential transcription factor that mediates cellular and systemic homeostatic responses to reduced oxygen availability in mammals. So far, using immunohistochemistry we have analyzed the association of HIF-1α expression with histological type among epithelial ovarian tumors. In the present study, quantitative analyses of activated HIF-1 level in the nucleus and of accumulated HIF-1α level in the cytoplasm were performed to clarify whether or not the hypoxic state would be correlated to histology, malignancy, and tumor size in epithelial ovarian tumors.
Method
HIF-1 level in the nucleus was analyzed using DNA binding assay, and HIF-1α level in the cytoplasm was measured by ELISA for a total of 36 epithelial ovarian tumors as follows: 5 serous adenocarcinomas (SEAs), 7 clear cell adenocarcinomas (CLAs), 7 endometrioid adenocarcinomas (ENAs), 4 mucinous adenocarcinomas (MUAs), 2 mucinous borderline tumors (MBTs), and 11 mucinous adenomas.
Results
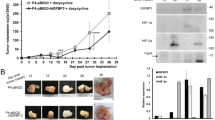
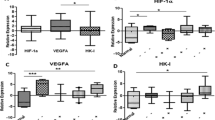
HIF-1 level (mg/ml) in the nucleus and HIF-1α level (mg/ml) in the cytoplasm were on average 0.116 and 0.178 for SEAs, 0.328 and 0.306 for CLAs, 0.171 and 0.305 for ENAs, 0.097 and 0.176 for MUAs, 0.224 and 0.180 for mucinous borderline tumors, 0.152 and 0.154 for mucinous adenomas. CLAs showed the highest levels for both of HIF-1 and HIF-1α, while MUAs showed the lowest levels of both. Mucinous adenomas were higher in HIF-1 than MUAs.
Conclusion
Hypoxic state was considered to be closely related to histological type of epithelial ovarian tumors, suggesting that CLAs may be most hypoxic. In the comparison of mucinous tumors, malignancies would not always become most hypoxic. Tumor size may not be strongly associated with hypoxic state.







Similar content being viewed by others
References
Jiang H, Feng Y (2006) Hypoxia-inducible factor 1α (HIF-1α) correlated with tumor growth and apoptosis in ovarian cancer. Int J Gynecol Cancer 16:405–412. doi:10.1111/j.1525-1438.2006.00310.x
Richard DE, Berra E, Pouyssegur J (1999) Angiogenesis: how a tumor adapts to hypoxia. Biochem Biophys Res Commun 266:718–722. doi:10.1006/bbrc.1999.1889
Jiang BH, Semenza GL, Bauer C, Marti HH (1996) Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 271:C1172–C1180
Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O’Rourke J, Mole DR et al (2001) C. elegan EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43–54. doi:10.1016/S0092-8674(01)00507-4
Semenza GL (2002) HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 8:S62–S67. doi:10.1016/S1471-4914(02)02317-1
Yasuda M, Shimizu M, Fujita M, Miyazawa M, Tang X, Kajiwara H et al (2007) Usefulness of hypoxia inducible factor-1 alpha in evaluating the prostatic adenocarcinoma viability following neoadjuvant hormone therapy. Cancer Detect Prev 31:396–401. doi:10.1016/j.cdp.2007.10.007
Mayer A, Höckel M, Vaupel P (2006) Endogenous hypoxia markers in locally advanced cancers of the uterine cervix: reality or wishful thinking? Strahlenther Onkol 182:501–510. doi:10.1007/s00066-006-1525-9
Sivridis E, Giatromanolaki A, Gatter KC, Harris AL, Koukourakis MI, Tumor and Angiogenesis Research Group (2002) Association of hypoxia-inducible factors 1α and 2α with activated angiogenic pathways and prognosis in patients with endometrial carcinoma. Cancer 95:1055–1063. doi:10.1002/cncr.10774
Birner P, Schindl M, Obermair A, Breitenecker G, Oberhuber G (2001) Expression of hypoxia-inducible factor 1α in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res 7:1661–1668
Osada R, Horiuchi A, Kikuchi N, Yoshida J, Hayashi A, Ota M, Katsuyama Y, Melillo G, Konishi I (2007) Expression of hypoxia-inducible factor 1alpha, hypoxia-inducible factor 2alpha, and von Hippel-Lindau protein in epithelial ovarian neoplasms and allelic loss of von Hippel-Lindau gene: nuclear expression of hypoxia-inducible factor 1alpha is an independent prognostic factor in ovarian carcinoma. Hum Pathol 38:1310–1320. doi:10.1016/j.humpath.2007.02.010
Miyazawa M, Yasuda M, Kaziwara H, Iida T, Fujita M, Hayashi M, et al. (2005) Expression of hypoxia-related markers, GLUT-1 and HIF-1 alpha, in the ovarian tumors: Association with histology and tumor grade. Acta His Chem 38:abstr S26
Yasuda M, Miyazawa M, Fujita M, Kajiwara H, Iida T, Hirasawa T et al (2008) Expression of hypoxia inducible factor-1· (HIF-1α) and glucose transporter-1 (GLUT-1) in ovarian adenocarcinomas: difference in hypoxic status depending on histological character. Oncol Rep 19:111–116
Iida T, Yasuda M, Miyazawa M, Fujita M, Osamura RY, Hirasawa T, Muramatsu T, Murakami M, Saito K, Mikami M (2008) Hypoxic status in ovarian serous and mucinous tumors: relationship between histological characteristics and HIF-1 alpha/GLUT-1 expression. Arch Gynecol Obstet 277:539–546. doi:10.1007/s00404-007-0500-8
Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T et al (2000) Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer 88:2584–2589. doi:10.1002/1097-0142(20000601)88:11<2584::AID-CNCR22>3.0.CO;2-5
Itamochi H, Kigawa J, Sugiyama T, Kikuchi Y, Suzuki M, Terakawa N (2002) Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstet Gynecol 100:281–287. doi:10.1016/S0029-7844(02)02040-9
Itamochi H, Kigawa J, Akeshima R, Sato S, Kamazawa S, Takahashi M et al (2002) Mechanisms of cisplatin resistance in clear cell carcinoma of the ovary. Oncology 62:349–353. doi:10.1159/000065067
Ho CM, Huang YJ, Chen TC, Huang SH, Liu FS, Chang Chien CC et al (2004) Pure-type clear cell carcinoma of the ovary as a distinct histological type and improved survival in patients treated with paclitaxel-platinum-based chemotherapy in pure-type advanced disease. Gynecol Oncol 94:197–203. doi:10.1016/j.ygyno.2004.04.004
Jiang H, Feng Y (2006) Hypoxia-inducible factor 1alpha (HIF-1alpha) correlated with tumor growth and apoptosis in ovarian cancer. Int J Gynecol Cancer 16(Suppl 1):405–412. doi:10.1111/j.1525-1438.2006.00310.x
Guertin DA, Sabatini DM (2005) An expanding role for mTOR in cancer. Trends Mol Med 11:353–361. doi:10.1016/j.molmed.2005.06.007
Bjornsti MA, Houghton PJ (2004) The TOR pathway: a target for cancer therapy. Nat Rev Cancer 4:335–348. doi:10.1038/nrc1362
Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F et al (2002) Regulation of hypoxia-inducible factor 1 alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22:7004–7014. doi:10.1128/MCB.22.20.7004-7014.2002
Kimbro KS, Simons JW (2006) Hypoxia-inducible factor-1 in human breast and prostate cancer. Endocr Relat Cancer 13:739–749. doi:10.1677/erc.1.00728
Sowter HM, Raval RR, Moore JW, Ratcliffe PJ, Harris AL (2003) Predominant role of hypoxia-inducible transcription factor (Hif)-1alpha versus Hif-2alpha in regulation of the transcriptional response to hypoxia. Cancer Res 63:6130–6134
Klatte T, Seligson DB, Riggs SB, Leppert JT, Berkman MK, Kleid MD et al (2007) Hypoxia-inducible factor 1 alpha in clear cell renal cell carcinoma. Clin Cancer Res 13:7388–7393. doi:10.1158/1078-0432.CCR-07-0411
Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK et al (2007) RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res 67:2408–2413. doi:10.1158/0008-5472.CAN-06-4490
Fujita M, Yasuda M, Kitatani K, Miyazawa M, Hirabayashi K et al (2007) An up-to-date anti-cancer treatment strategy focusing on HIF-1alpha suppression: its application for refractory ovarian cancer. Acta Histochem Cytochem 40:139–142. doi:10.1267/ahc.07024
Yeo EJ, Chun YS, Park JW (2004) New anticancer strategies targeting HIF-1. Biochem Pharmacol 68:1061–1069. doi:10.1016/j.bcp.2004.02.040
Lee S, Garner EI, Welch WR, Berkowitz RS, Mok SC (2007) Over-expression of hypoxia-inducible factor 1 alpha in ovarian clear cell carcinoma. Gynecol Oncol 106:311–317. doi:10.1016/j.ygyno.2007.03.041
Dang DT, Chun SY, Burkitt K, Abe M, Chen S, Havre P et al (2008) Hypoxia-inducible factor-1 target genes as indicators of tumor vessel response to vascular endothelial growth factor inhibition. Cancer Res 68:1872–1880. doi:10.1158/0008-5472.CAN-07-1589
Takahashi Y, Nishikawa M, Takakura Y (2008) Inhibition of tumor cell growth in the liver by RNA interference-mediated suppression of HIF-1alpha expression in tumor cells and hepatocytes. Gene Ther 15:572–582. doi:10.1038/sj.gt.3303103
Amornphimoltham P, Patel V, Leelahavanichkul K, Abraham RT, Gutkind JS (2008) A retroinhibition approach reveals a tumor cell-autonomous response to rapamycin in head and neck cancer. Cancer Res 68:1144–1153. doi:10.1158/0008-5472.CAN-07-1756
Jiang HY, Feng YJ (2004) Inhibition of hypoxia-inducible factor 1alpha expression and tumor growth in SKOV3 ovarian cancer model by sirolimus. Zhonghua Fu Chan Ke Za Zhi 39:474–477
Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR et al (2004) Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol 22:909–918. doi:10.1200/JCO.2004.08.185
Sosman JA, Puzanov I, Atkins MB (2007) Opportunities and obstacles to combination targeted therapy in renal cell cancer. Clin Cancer Res 13:764s–769s. doi:10.1158/1078-0432.CCR-06-1975
Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH et al (2007) RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res 13:4261–4270. doi:10.1158/1078-0432.CCR-06-2770
Conflict of interest statement
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyazawa, M., Yasuda, M., Fujita, M. et al. Association of hypoxia-inducible factor-1 expression with histology in epithelial ovarian tumors: a quantitative analysis of HIF-1. Arch Gynecol Obstet 279, 789–796 (2009). https://doi.org/10.1007/s00404-008-0816-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-008-0816-z