Abstract
Purpose/objective(s)
Cervical cancer is one of the deadliest cancers in women with a death toll of 230,000 worldwide each year, nearly 80% in developing countries. Radiotherapy (RT) is a major treatment modality for advanced cervical cancer but the local relapse rate is 30–44% in patients treated with RT alone and 19–25% in patients treated with concurrent chemoradiotherapy. Previous studies have shown that the transcription factor NF-κB is constitutively expressed in human cervical squamous cell carcinomas. NF-κB activation also contributes to the resistance of cervical cancer cells to apoptosis induced by chemotherapeutic agents and radiation. Therefore, inhibition of NF-κB in tumor cells may render them more sensitive to chemo/radiation therapies. The objective of this study is to investigate the potential of radiosensitization of NF-κB inhibition by Velcade in human cervical cancer cell lines.
Materials and methods
We used the human cervical cancer cell lines HeLa and SiHa. Both are highly radioresistant and chemoresistant as compared to other cervical cancer cell lines. These cells had been treated with Velcade before they were irradiated with different doses of ionizing radiation. MTT metabolic assays and clonogenic cell survival assays were performed to evaluate the effects of Velcade on radiation resistance.
Results
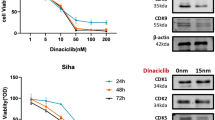
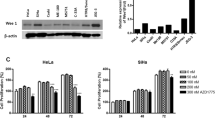
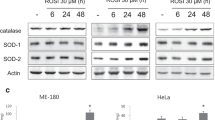
Inhibition of NF-κB by Velcade alone decreased metabolic potential (MTT) and clonogenic survival in SiHa, but not in HeLa cells. Furthermore, pre-treatment of SiHa, but not HeLa cells with Velcade enhanced radiation sensitivity.
Conclusions
Inhibition of NF-κB by the proteasome inhibitor Velcade enhances radiosensitivity of certain human cervical carcinoma cancer cells in vitro. These results raise the possibility that inhibition of NF-κB will result in radiosensitization only in those tumor cells which are more dependent on NF-κB for their metabolism and survival, however, the radiosensitivity of “NF-κB independent” cells are not likely influenced by it.






Similar content being viewed by others
References
Gilmore TD (2006) Introduction to NF-κB: players, pathways, perspectives. Oncogene 25:6680–6684
Pikarsky E, Porat RM, Stein I et al (2004) NF-κB functions as a tumor promoter in inflammation-associated cancer. Nature 431:461–466
Greten FR, Eckmann L, Greten TF et al (2004) IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118:285–296
Ren Q, Kari C, Quadros MR et al (2006) Malignant transformation of immortalized HaCaT keratinocytes through deregulated nuclear factor κB signaling. Cancer Res 66(10):5209–5215
Cusack J, Liu R, Badwin A (1999) NFκB and chemoresistance: potentiation of cancer chemotherapy via inhibition of NFκB. Drug Resist Updat 2:271–273
Gilmore TD, Herscovitch M (2006) Inhibitors of NF-κB signaling. Oncogene 25:6887–6899
Adams J, Palombella VJ, Sausville EA et al (1999) Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res 59:2615–2622
Wang CY, Cusack J, Liu R et al (1999) Control of inducible chemoresistance enhanced anti-tumor therapy through increased apoptosis by inhibition of NFκB. Nature Medicine 4:412–417
Sunwoo JB, Chen Z, Dong G et al (2001) Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kB, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clinical Cancer Res 7:1419–1428
Williams S, Pettaway C, Song R et al (2003) Differential effects of the proteasome inhibitor bortezomib on apoptosis and angiogenesis in human prostate tumor xenografts. Mol Cancer Ther 2:835–843
Cardoso F, Durbecq V, Laes JF et al (2006) Bortezomib (PS-341, Velcade) increases the efficacy of trastuzumab (Herceptin) in HER-2-positive breast cancer cells in a synergistic manner. Mol Cancer Ther 5(12):3042–3051
Goel A, Dispensieri A, Greipp PR et al (2005) PS-341-mediated selective targeting of multiple myeloma cells by synergistic increase in ionizing radiation-induced apoptosis. Exp Hematol 33:784–795
Eifel PJ (2006) Chemoradiotherapy in the treatment of cervical cancer. Semin Radiat Oncol 16(3):177–185
Nair A, Venkatraman M, Maliekal TT et al (2003) NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene 22(1):50–58
Bradbury M, Markovina S, Wei SJ (2001) Indomethacin-induced radiosensitization and inhibition of ionizing radiation-induced NF-κB activation in HeLa Cells Occur via a mechanism involving p38 MAP Kinase. Cancer Res 61:7689–7696
Venkatraman M, Anto RJ, Nair A et al (2005) Biological and chemical inhibitors of NF-kappaB sensitize SiHa cells to cisplatin-induced apoptosis. Mol Carcinog 44(1):51–9
Gurska S, Farkasova T, Gabelova A (2007) Radiosensitivity of cervical cancer cell lines: the impact of polymorphisms in DNA repair genes. Neoplasm 54(3):195–201
Saxena A, Yashar C, Taylor DD et al (2005) Cellular response to chemotherapy and radiation in cervical cancer. Am J Obstet Gynecol 192:1399–1403
Liu SS, Chan LYY, Leung RCY et al (2006) Enhancement of radiosensitivity of cervical cancer cells by overexpressing p73α. Mol Cancer Ther 5(5):1209–1215
Fogh J, Fogh JM, Orfeo T (1977) One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst 59(1):221–226
Ahmed KM, Li JJ (2008) NF-Kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med 44(1):1–13
Chuang SE, Yeh PY, Lu YS et al (2002) Basal levels and patterns of anticancer drug-induced activation of NF-κB and its attenuation by tamoxifen, dexamethansone, and curcumin in carcinoma cells. Biochem Pharmacol 63:1709–1716
Shukla S, Gupta S. (2004) Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor (NF)-kappaB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-kappaB-responsive genes. Clin Cancer Res 10(9):3169–3178
Mishra A, Bharti AC, Varghese P et al (2006) Differential expression and activation of NF-kappaB family proteins during oral carcinogenesis: Role of high risk human papillomavirus infection. Int J Cancer 119(12):2840–2850
Didelot C, Heyob MB, Bianchi A et al (2001) Constitutive NF-κB activity influences basal apoptosis and radiosensitivity of head-and-neck carcinoma cell lines. Int J Radiat Oncol Biol Phys 51:1354–1360
Wang CY, Mayo MW, Baldwin AS et al (1996) TNF-α and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274:784–787
Munshi A, Kurland JF, Nishikawa T et al (2004) Inhibition of constitutively activated nuclear factor-kappaB radiosensitizes human melanoma cells. Mol Cancer Ther 3(8):985–992
Mendonca MS, Chin-Sinex H, Gomez-Millan J et al (2007) Parthenolide sensitizes cells to X-ray-induced cell killing through inhibition of NF-kappaB and split-dose repair. Radiat Res 168(6):689–697
Ciechanover A (1998) The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J17:7151–7160
Adams J (2004) The proteasome: a suitable antineoplastic target. Nat Rev Cancer 4:349–360
Michaelis M, Fichtner I, Behrens D et al (2006) Anti-cancer effects of bortezomib against chemoresistant neuroblastoma cell lines in vitro and in vivo. Int J Oncol 28(2):439–446
Russo SM, Tepper JE, Baldwin AS Jr et al (2001) Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. Int J Radiat Oncol Biol Phys 50(1):183–193
Belka C (2006) The fate of irradiated tumor cells. Oncogene 25(7):969–971
Jacquemont C, Taniguchi T (2007) Proteasome function is required for DNA damage response and fanconi anemia pathway activation. Cancer Res 67(15):7395–7405
Aghajanian C, Soignet S, Dizon DS et al (2002) A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res 8(8):2505–2511
Dreicer R, Petrylak D, Agus D et al (2007) Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin Cancer Res 13(4):1208–1215
Berenson JR, Yang HH, Sadler K et al (2006) Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol 24(6):937–944
Schmid P, Kühnhardt D, Kiewe P et al (2008) A phase I/II study of bortezomib and capecitabine in patients with metastatic breast cancer previously treated with taxanes and/or anthracyclines. Ann Oncol [Epub ahead of print]
Lee TL, Yang XP, Yan B (2007) A novel nuclear factor-κB gene signature is differentially expressed in head and neck squamous cell carcinomas in association with TP53 status. Clin Cancer Res 13(19):5680–5691
Lorch JH, Thomas TO, Schmoll HJ (2007) Bortezomib inhibits cell-cell adhesion and cell migration and enhances epidermal growth factor receptor inhibitor-induced cell death in squamous cell cancer. Cancer Res 67:727–734
Acknowledgements
We thank Dr. YaPing Sui for her technical assistance and Dr. Csaba Kari for his help on preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamer, S., Ren, Q. & Dicker, A.P. Differential radiation sensitization of human cervical cancer cell lines by the proteasome inhibitor velcade (bortezomib, PS-341). Arch Gynecol Obstet 279, 41–46 (2009). https://doi.org/10.1007/s00404-008-0667-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-008-0667-7