Abstract
Introduction
Galectin-1 (Gal-1), a carbohydrate-binding protein, is differentially expressed by various normal and pathological tissues and appears to be functionally polyvalent. Recent evidence indicates that Gal-1 is involved in the proliferation of adult neural progenitor cells in neurogenic regions during adulthood. However, localization and functional roles of Gal-1 in the adult spinal cord have not been clarified.
Method
Here, we investigated the spatio-temporal profile of endogenous Gal-1 expression by in situ hybridization before and after experimental adult spinal cord injury and examined the correlation of Gal-1 with the fate of dividing cells in vivo, using double-labeling methods. Gal-1 mRNA was detectable at a relatively low level in uninjured spinal cord, but was markedly increased in the gray matter and/or white matter and in the ependyma rostral and caudal to the lesion site after injury.
Results
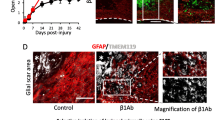
Co-localization results revealed that Gal-1 was expressed predominantly by GFAP-positive reactive astrocytes. In addition, intrathecal infusion of recombinant Gal-1 enhanced cell division and reactive astrocytosis in the adult spinal cord. To explore further whether Gal-1 and reactive astrocytes provide a synergistic effect on neurological recovery following SCI, we investigated the differences in behavioral analysis between wild-type (WT) and reactive astrocyte-deficient transgenic mice after injury and found neuroprotective effects of Gal-1 appeared to be specifically mediated through reactive astrocytes.
Conclusion
These results indicate that Gal-1 exhibits great potential as a novel neuroprotective agent for the treatment of SCI.





Similar content being viewed by others
References
Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, Thal LJ, Gage FH (2000) Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci 20:2218–2228
Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M (2001) Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol 172:115–127
Takahashi M, Arai Y, Kurosawa H, Sueyoshi N, Shirai S (2003) Ependymal cell reactions in spinal cord segments after compression injury in adult rat. J Neuropathol Exp Neurol 62:185–194
Barondes SH, Cooper DN, Gitt MA, Leffler H (1994) Galectins: structure and function of a large family of animal lectins. J Biol Chem 269:20807–20810
Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K (1994) Galectins: a family of animal beta-galactoside-binding lectins. Cell 76:597–598
Camby I, Mercier ML, Lefranc F, Kiss R (2006) Galectin-1: a small protein with major functions. Glycobiology 16:137–157
Inagaki Y, Sohma Y, Horie H, Nozawa R, Kadoya T (2000) Oxidized galectin-1 promotes axonal regeneration in peripheral nerves but does not possess lectin properties. Eur J Biochem 267:2955–2964
Chang-Hong R, Wada M, Koyama S, Kimura H, Arawaka S, Kawanami T, Kurita K, Kadoya T, Aoki M, Itoyama Y (2005) Neuroprotective effect of oxidized galectin-1 in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol 194:203–211
Horie H, Inagaki Y, Sohma Y, Nozawa R, Okawa K, Hasegawa M, Muramatsu N, Kawano H, Horie M, Koyama H (1999) Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J Neurosci 19:9964–9974
Horie H, Kadoya T, Hikawa N, Sango K, Inoue H, Takeshita K, Asawa R, Hiroi T, Sato M, Yoshioka T (2004) Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci 24:1873–1880
Sakaguchi M, Shingo T, Okano HJ, Shiwa M, Okano H (2006) A carbohydrate-binding protein, galectin-1, promotes proliferation of adult neural stem cells. Proc Natl Acad Sci USA 103:7112–7117
Akazawa C, Nakamura Y, Sango K, Horie H, Kohsaka S (2004) Distribution of the galectin-1 mRNA in the rat nervous system: its transient upregulation in rat facial motor neurons after facial nerve axotomy. Neuroscience 125:171–178
Slezak M, Pfrieger FW (2003) New roles for astrocytes: regulation of CNS synaptogenesis. Trends Neurosci 26:531–535
Doetsch F, García-Verdugo JM, Alvarez-Buylla A (1997) Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 17:5046–5061
Sofroniew MV (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11:400–407
Fischer AJ, McGuire CR, Dierks BD, Reh TA (2002) Insulin and fibroblast growth factor 2 activate a neurogenic program in Müller glia of the chicken retina. J Neurosci 22:9387–9398
Goutan E, Martí E, Ferrer I (1998) BDNF, and full length and truncated TrkB expression in the hippocampus of the rat following kainic acid excitotoxic damage. Evidence of complex time-dependent and cell-specific responses. Mol Brain Res 59:154–164
Sasaki T, Hirabayashi J, Kasai KI, Endo T (2004) Galectin-1 induces astrocyte differentiation, which leads to production of brain-derived neurotrophic factor. Glycobiology 14:357–363
Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV (1998) Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93:189–201
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucker L, Johnson MH, Sofroniew MV (1999) Leukocyte infiltration, neuronal degeneration and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23:297–308
Lee Y, Su M, Messing A, Brenner M (2006) Astrocyte heterogeneity revealed by expression of a GFAP-LacZ transgene. Glia 53:677–687
Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE (2003) Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 20:179–193
Hayes CS, Mulkmus SA, Cizkova D, Yaksh TL, Hua XY (2003) A double-lumen intrathecal catheter for studies of modulation of spinal opiate tolerance. J Neurosci Methods 126:165–173
Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transaction. Exp Neurol 139:244–256
Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26:523–530
Seri B, Garcia-Verdugo JM, Alvarez-Buylla A (2004) Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol 478:359–378
Wu XH, Yang SH, Duan DY, Bao YT, Zhang Y (2007) Anti-apoptotic effect of insulin in the control of cell death and neurologic deficit after acute spinal cord injury in mice. J Neurotrauma 24:1502–1512
Radojicic M, Nistor G, Keirstead HS (2007) Ascending central canal dilation and progressive ependymal disruption in a contusion model of rodent chronic spinal cord injury. BMC Neurol 30:1481–1492
He J, Baum LG (2004) Presentation of galectin-1 by extracellular matrix trigger T cell death. J Biol Chem 279:4705–4712
Campos LS, Decker L, Taylor V, Skarnes W (2006) Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J Biol Chem 281:5300–5309
Iseda T, Nishio T, Kawaguchi S, Yamanoto M, Kawasaki T, Wakisaka S (2004) Spontaneous regeneration of the corticospinal tract after transection in young rats: a key role of reactive astrocytes in making favorable and unfavorable conditions for regeneration. Neuroscience 126:365–374
Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H (2006) Conditional ablation of STAT3/SOCS3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834
Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44
Barkho BZ, Song H, Gage FH (2006) Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cells Dev 15:407–421
Azari MF, Profyris C, Zang DW, Petratos S, Cheema SS (2005) Induction of endogenous neural precursors in mouse models of spinal cord injury and disease. Eur J Neurol 12:638–648
Emirandetti A, Graciele Zanon R, Sabha M, de Oliveira AL (2006) Astrocyte reactivity influences the number of presynaptic terminals apposed to spinal motoneurons after axotomy. Brain Res 1095:35–42
Widestrand A, Faijerson J, Wilhelmsson U, Smith PL, Li L, Sihlbom C, Eriksson PS, Pekny M (2007) Increased neurogenesis and astrogenesis from neural progenitor cells grafted in the hippocampus of GFAP−/− Vim−/− mice. Stem Cells 25:2619–2627
Imura T, Kornblum HI, Sofroniew MV (2003) The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci 23:2824–2832
Imura T, Nakano I, Kornblum HI, Sofroniew MV (2006) Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia 53:277–293
Jalabi W, Boehm N, Grucker D, Ghandour MS (2005) Recovery of myelin after induction of oligodendrocyte cell death in postnatal brain. J Neurosci 25:2885–2894
Gowing G, Vallières L, Julien JP (2006) Mouse model for ablation of proliferating microglia in acute CNS injuries. Glia 53:331–337
Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J (2007) Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci 27:2596–2605
Acknowledgments
Supported by National Nature Science Foundation of China (No. 30800333). The authors are very grateful to Cuifang Wang for her skilled technical assistance with histological processing and Jing Wang for assistance with behavioral tests.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, H., Xia, Y., Wang, S. et al. Synergistic effects of galectin-1 and reactive astrocytes on functional recovery after contusive spinal cord injury. Arch Orthop Trauma Surg 131, 829–839 (2011). https://doi.org/10.1007/s00402-010-1233-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-010-1233-x