Abstract
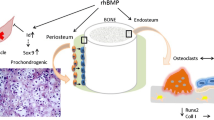
Bone morphogenic proteins (BMPs) are pleiotropic regulators of bone volume, skeletal organogenesis and bone regeneration after a fracture. They function as signaling agents to affect cellular events like proliferation, differentiation and extracellular matrix synthesis. Clinically utilized rhBMP-2 combines rhBMP-2 with an osteoconductive carrier to induce bone growth and acts as a bone graft substitute. rhBMP-2, initially released in 2002, has been used primarily in spinal fusions in the lumbar and cervical regions. Recently, the application of rhBMP-2 has extended into the orthopedic trauma setting with increased application in open tibia fractures. This review outlines the history of development, molecular characteristics, toxicity and clinical applications.
Similar content being viewed by others
References
Riley EH, Lane JM, Urist MR et al (1996) Bone morphogenetic protein-2: biology and applications. Clin Orthop Relat Res 324:39–46. doi:10.1097/00003086-199603000-00006
Urist MR (1965) Bone: formation by autoinduction. Science 150:893–899. doi:10.1126/science.150.3698.893
Urist MR, Iwata H, Ceccotti PL et al (1973) Bone morphogenesis in implants of insoluble bone gelatin. Proc Natl Acad Sci USA 70:3511–3515. doi:10.1073/pnas.70.12.3511
McKay D, Sandhu HS (2002) Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine 27(16S):S66–S85. doi:10.1097/00007632-200208151-00014
Reddi AH (1998) Initiation of fracture repair by bone morphogenetic proteins. Clin Orthop Relat Res 355S:s66–s72. doi:10.1097/00003086-199810001-00008
Lind M (1998) Growth factor stimulation of bone healing. Effect on osteoblasts, osteotomies, and implants fixation. Acta Orthop Scand 69(Suppl):283
Ludwig SC, Boden SD (1999) Osteoinductive bone graft substitutes for spinal fusion. A basic science summary. Orthop Clin North Am 30(4):635–645
Ebara S, Nakayama K (2002) Mechanism for the action of bone morphogenetic proteins and regulation of their activity. Spine 27(16S):S10–S15. doi:10.1097/00007632-200208151-00004
Helm G, Anderson G, Andersson GBJ et al (2002) Summary statement: bone morphogenetic proteins. Spine 27(16S):S9. doi:10.1097/00007632-200208151-00003
Wozney JM (2002) Overview of bone morphogenetic proteins. Spine 27(16S):S2–S8. doi:10.1097/00007632-200208151-00002
Schaefer SL, Lu Y, Seeherman H et al (2008) Effect of rhBMP-2 on tibial plateau fractures in a canine model. J Orthop Res
Glassman SD, Dimar JR, Carreon LY et al (2005) Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine 30(15):1694–1698. doi:10.1097/01.brs.0000172157.39513.80
Yoon ST, Boden SD (2002) Osteoinductive molecules in orthopaedics: basic science and preclinical studies. Clin Orthop Relat Res (395):33–43. doi:10.1097/00003086-200202000-00005
Israel DI, Nove J, Kerns KM et al (1992) Expression and characterization of bone morphogenetic protein-2 in Chinese hamster ovary cells. Growth Factors 7:139–150. doi:10.3109/08977199209046403
McKay W (2001) Medtronic Sofamore Danek. Personal communication. Study on file with FDA, Rockville
Poynton AR, Lane JM (2002) Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine 27(16S):S40–S48. doi:10.1097/00007632-200208151-00010
Martin GJ Jr, Boden SD, Morone MA et al (1999) Posterolateral intertransverse process spinal arthrodesis with rhBMP-2 in a nonhuman primate: important lessons learned regarding dose, carrier, and safety. J Spinal Disord 12(3):179–186
Sandhu HS, Kanim LE, Kabo JM et al (1995) Evaluation of rhBMP-2 with an OPLA carrier in a canine posterolateral (transverse process) spinal fusion model. Spine 20(24):2669–2682. doi:10.1097/00007632-199512150-00008
Meyer RA Jr, Gruber HE, Howard BA et al (1999) Safety of recombinant human bone morphogenetic protein-2 after spinal laminectomy in the dog. Spine 24(8):747–754. doi:10.1097/00007632-199904150-00004
David SM, Gruber HE, Meyer RA Jr et al (1999) Lumbar spinal fusion using recombinant human bone morphogenetic protein in the canine. A comparison of three dosages and two carriers. Spine 24(19):1973–1979. doi:10.1097/00007632-199910010-00002
Boden SD, Martin GJ Jr, Morone MA et al (1999) Posterolateral lumbar intertransverse process spine arthrodesis with recombinant human bone morphogenetic protein 2/hydroxyapatite-tricalcium phosphate after laminectomy in the nonhuman primate. Spine 24(12):1179–1185. doi:10.1097/00007632-199906150-00002
Valentin-Opran A, Wozney J, Csimma C et al (2002) Clinical evaluation of recombinant human bone morphogenetic protein-2. Clin Orthop Relat Res (395):110–120. doi:10.1097/00003086-200202000-00011
Wang EA, Rosen V, D’Alessandro JS et al (1990) Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci USA 87:2220–2224. doi:10.1073/pnas.87.6.2220
Balemans W, Van Hul W (2001) Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol 250:231–250
Canalis E, Economides AN, Gazzerro E (2003) Bone morphogenetic proteins, their antagonists and the skeleton. Endocr Rev 24(2):218–235. doi:10.1210/er.2002-0023
Sandhu HS, Kanim LE, Kabo JM et al (1996) Effective doses or recombinant bone morphogenetic protein-2 in experimental spinal fusion. Spine 21(18):2115–2122. doi:10.1097/00007632-199609150-00012
Schimandle JH, Boden SD, Hutton WC (1995) Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine 20(12):1326–1337. doi:10.1097/00007632-199506000-00002
Boden SD, Schimandle JH, Hutton WC et al (1995) 1995 Volvo Award in basic sciences. The use of an osteoinductive growth factor for lumbar spinal fusion. Part I: Biology of spinal fusion. Spine 20(24):2626–2632
Boden SD, Schimandle JH, Hutton WC (1995) 1995 Volvo Award in basic sciences. The use of an osteoinductive growth factor for lumbar spinal fusion. Part II: Study of dose, carrier, and species. Spine 20(24):2633–2644
Ruppert R, Hoffman E, Sebald W (1996) Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem 237(1):295–302. doi:10.1111/j.1432-1033.1996.0295n.x
Sandhu HS, Toth JM, Diwan AD et al (2002) Histologic evaluation of the efficacy of rhBMP-2 compared with autograft bone in sheep spinal anterior interbody fusion. Spine 27:567. doi:10.1097/00007632-200203150-00003
Zdeblick TA, Ghanayem AJ, Rapoff AJ et al (1998) Cervical interbody fusion cage: an animal model with and without bone morphogenetic protein. Spine 23:758–766. doi:10.1097/00007632-199804010-00002
Murakami N, Saito N, Horiuchi H et al (2002) Repair of segmental defects in rabbit humeri with titanium fiber mesh cylinders containing recombinant human bone morphogenetic protein-2 (rhBMP-2) and a synthetic polymer. J Biomed Mater Res 62(2):169–174. doi:10.1002/jbm.10236
Lieberman JR, Daluiski A, Stevenson S et al (1999) The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am 81(7):905–917
Szpalski M, Gunzburg R (2005) Recombinant human bone morphogenetic protein-2: a novel osteoinductive alternative to autogenous bone graft? Acta Orthop Belg 71(2):133–148
Gerhart TN, Kirker-Head CA, Kriz MJ et al (1993) Healing segmental femoral defects in sheep using recombinant human bone morphogenetic protein. Clin Orthop Relat Res (293):317–326
Singh K, Smucker JD, Boden SD (2006) Use of recombinant human bone morphogenetic protein-2 as an adjunct in posterolateral lumbar spine fusion: a prospective CT-scan analysis at one and two years. J Spinal Disord Tech 19(6):416–423. doi:10.1097/00024720-200608000-00008
Dimar JR, Glassman SD, Burkus KJ et al (2006) Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine 31(22):2534–2539. doi:10.1097/01.brs.0000240715.78657.81 discussion 2540
Glassman SD, Carreon L, Djurasovic M et al (2007) Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J 7(1):44–49. doi:10.1016/j.spinee.2006.06.381
Haid RW Jr, Branch CL Jr, Alexander JT et al (2004) Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J 4(5):527–538. doi:10.1016/j.spinee.2004.03.025 discussion 538–539
Villavicencio AT, Burneikiene S, Nelson EL et al (2005) Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine 3(6):436–443
Lanman TH, Hopkins TJ (2004) Lumbar interbody fusion after treatment with recombinant human bone morphogenetic protein-2 added to poly(L-lactide-co-D, L-lactide) bioresorbable implants. Neurosurg Focus 16(3):E9
Mummaneni PV, Pan J, Haid RW, Rodts GE (2004) Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004
Lewandrowski K, Nanson C, Calderon R (2007) Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J 7(5):609–614. doi:10.1016/j.spinee.2007.01.011
Burkus JK, Gornet MF, Dickman C et al (2002) Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord 15:337–349
Burkus JK, Heim SE, Gornet MF et al (2003) Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech 16(2):113–122
Burkus JK, Dorchak JD, Sanders DL (2003) Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine 28(4):372–377. doi:10.1097/00007632-200302150-00012
Kleeman TJ, Ahn UM, Talbot-Kleeman A (2001) Laparoscopic anterior lumbar interbody fusion with rhBMP-2: a prospective study of clinical and radiographic outcomes. Spine 26:2751–2756. doi:10.1097/00007632-200112150-00026
Baskin DS, Ryan P, Sonntag V et al (2003) A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine 28(12):1219–1224. doi:10.1097/00007632-200306150-00003 discussion 1225
Vaidya R, Carp J, Sethi A et al (2007) Complications of anterior cervical discectomy and fusion using recombinant human bone morphogeneticprotein-2. Eur Spine J
Jones AL, Bucholz RW, Bosse MJ et al (2006) Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am 88(7):1431–1441. doi:10.2106/JBJS.E.00381
Nordsletten L (2006) Recent developments in the use of bone morphogenetic protein in orthopaedic trauma surgery. Curr Med Res Opin 22(Suppl 1):S13–S17 S23
Karladani AH, Granhed H, Karrholm J et al (2001) The influence of fracture etiology and type on fracture healing: a review of 104 consecutive tibial shaft fractures. Arch Orthop Trauma Surg 121(6):325–328. doi:10.1007/s004020000252
Bhandari M, Tornetta P 3rd, Sprague S et al (2003) Predictors of reoperation following operative management of fractures of the tibial shaft. J Orthop Trauma 17(5):353–361. doi:10.1097/00005131-200305000-00006
Riedel GE, Valentin-Opran A (1999) Clinical evaluation of rhBMP-2/ACS in orthopedic trauma: a progress report. Orthopedics 22(7):663–665
Govender S, Csimma C, Genant HK et al (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 84-A(12):2123–2134
Alt V, Heissel A (2006) Economic considerations for the use of recombinant human bone morphogenetic protein-2 in open tibial fractures in Europe: the German model. Curr Med Res Opin 22(Suppl 1):S19–S22. doi:10.1185/030079906X80602
Swiontkowski MF, Aro HT, Donell S et al (2006) Recombinant human bone morphogenetic protein-2 in open tibial fractures. A subgroup analysis of data combined from two prospective randomized studies. J Bone Joint Surg Am 88(6):1258–1265. doi:10.2106/JBJS.E.00499
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valdes, M.A., Thakur, N.A., Namdari, S. et al. Recombinant bone morphogenic protein-2 in orthopaedic surgery: a review. Arch Orthop Trauma Surg 129, 1651–1657 (2009). https://doi.org/10.1007/s00402-009-0850-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-009-0850-8