Abstract
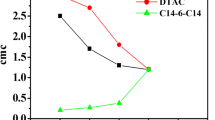
Surface-active ionic liquids (SAILs) can modify the physiological properties of conventional surfactants. Mixed micelles of isoquinoline-based SAILs, dodecylisoquinolinium bromide [C12iQuin][Br], tetradecylisoquinolinium bromide [C14iQuin][Br] and cationic surfactant, dodecyltrimethylammonium bromide (DTAB) in aqueous medium and their interactions have been studied by employing conductometry and 1H NMR technique. The critical micelle concentration (cmc) and various thermodynamic parameters have been calculated from conductometry measurements. Mixed micellar parameters such as ideal cmc (cmc*), micellar mole fraction (\( {X}_1^m \)), micellar interaction parameter (βm) and activity coefficients (f1) and (f2) of component 1 (SAIL) and component 2 (DTAB) have been evaluated by applying Clint, Rubingh and Motomura theoretical models. The interaction between SAILs and surfactant has been found synergistic and non-ideal. The peak merging and broadening of protons suggests the growth of micelles. Use of SAILs as an additive effectively reduce the cmc that is indicated by negative value of interaction parameter (βm).






Similar content being viewed by others
References
Welton T (1999) Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99(8):2071–2084. https://doi.org/10.1021/cr980032t
Dupont J, De Souza RF, Suarez PAZ (2002) Ionic liquid (molten salt) phase organometallic catalysis. Chem Rev 102(10):3667–3692. https://doi.org/10.1021/cr010338r
Seddon KR (2003) Ionic liquids: a taste of future. Nature 2:360–365
Mehnert CP, Cook RA, Dispenziere NC, Afeworki M (2002) Supported ionic liquid catalysis—a new concept for homogeneous hydroformylation catalysis. J Am Chem Soc 124(44):12932–12933. https://doi.org/10.1021/ja0279242
Wilkes JS (2002) A short history of ionic liquids- from molten salts to neoteric solvents. Green Chem 73(4):73–80. https://doi.org/10.1039/B110838G
Earle MJ, Esperanca JMSS, Gilea MA, Lopes JNC, Rebelo LPN, Maggee JW, Seddon KR, Widegren JA (2006) The distillation and volatility of ionic liquids. Nature 439:831–834. https://doi.org/10.1038/nature04451
Mahajan S, Sharma R, Mahajan RK (2013) Surface adsorption and mixed micelle formation of surface active ionic liquid in cationic surfactants: conductivity, surface tension, fluorescence and NMR studies. Colloids Surf A Physicochem Eng Asp 427:62–75. https://doi.org/10.1016/j.colsurfa.2013.03.023
Shi L, Jing X, Gao H, Gu Y, Zheng L (2013) Ionic liquid induced changes in the properties of aqueous sodium sulfate solution: effect of acidic/basic functional groups. Colloid Polym Sci 291:1601–1612. https://doi.org/10.1007/s00396-013-2894-0
Cognigni A, Gaertner P, Zirbs R, Peterlik H, Prochazka K, Schroder C, Bica K (2016) Surface-active ionic liquids in micellar catalysis: impact of anion selection on reaction rates in nucleophilic substitutitions. Phys Chem Chem Phys 18:13375–13384. https://doi.org/10.1039/C6CP00493H
Eber J, Wasserscheid P, Jess A (2004) Deep desulfurization of oil refinery streams by extraction with ionic liquids. Green Chem 6:316–322. https://doi.org/10.1039/B407028C
Pendletton JN, Gilmore PB (2015) The antimicrobial potential of ionic liquids: a source of chemical diversity for infection and biofilm control. Int J Antimicrob Agents 46(2):131–139. https://doi.org/10.1016/j.ijantimicag.2015.02.016
Cornellas A, Perez L, Comelles F, Ribosa I, Manresa A, Teresa GM (2011) Self-aggregation and antimicrobial activity of imidazolium and pyridinium based ionic liquids in aqueous solution. J Colloid Interface Sci 355(1):164–171. https://doi.org/10.1016/j.jcis.2010.11.063
Culter WG, Kirsa E (1987) Detergency: theory and practical. Marcel Dekker, New York
Salwa MIM (2014) Role of surfactants in nanotechnology and their applications. Int J Curr Microbiol App Sci 3(5):237–260
Reiger MM (1985) Surfactants in cosmetics. Marcel Dekker, New York
Hemmateeenejad B, Safavai A, Dorostkar S (2011) Aggregation of imidazolium based ionic liquids in binary methanol-water solvents: a linear solvation free energy relationship study. J Mol Liq 160:35–39. https://doi.org/10.1016/j.molliq.2011.02.011
Chabba S, Kumar S, Aswal VK, Kang TS, Mahajan RK (2015) Interfacial and aggregation behaviour of aqueous mixtures of imidazolium based surface active ionic liquids and anionic surfactants sodium dodecylbenzenesulfonate. Colloids Surf A Physicochem Eng Asp 472:9–20. https://doi.org/10.1016/j.colsurfa.2015.02.032
Comelles F, Ribosa I, Gonzalez JJ, Garcia MT (2015) Catanionic surfactant formation from the interaction of the cationic surfactant hexadecyltrimethylammonium bromide (CTAB) and the ionic liquid 1-butyl-3-methylimidazolium octylsulfate (bmim-octyl SO4) in aqueous solution. Colloids Surf A Physicochem Eng Asp 484:136–143. https://doi.org/10.1016/j.colsurfa.2015.07.051
Smirnova NA, Vanin AA, Safonova EA, Pukinsky IB, Anufrikov YA, Makarov AL (2009) Self-assembly in aqueous solutions of imidazolium ionic liquids and their mixtures with an anionic surfactant. J Colloid Interface Sci 336:793–802. https://doi.org/10.1016/j.jcis.2009.04.004
Thakkar K, Bharatiya B, Shah DO, Ray D, Aswal VK, Bahadur P (2015) Interaction of ionic liquid type cationic surfactants with triton X-100 nonionic micelles. Colloids Surf A Physicochem Eng Asp 484:547–557. https://doi.org/10.1016/j.colsurfa.2015.08.039
Nagarajan R (1985) Molecular theory for mixed micelles. Langmuir 1:331–341. https://doi.org/10.1021/la00063a012
Wang XH, Qin L (2017) Surface adsorption and thermodynamic properties of mixed systems of ionic liquid surfactants with cetyltrimethylammonium bromide. RSC Adv 7:51426–51435. https://doi.org/10.1039/c7ra08915e
Zheng P, Yin H, Zhao J, Shen W (2016) Measurements of the interaction enthalpy for mixed micelles of dodecyltrimethylammonium bromide and 1-dodecyl-3-methylimidazolium bromide. J Ind Eng Chem 44:204–209. https://doi.org/10.1016/j.jiec.2016.09.001
Zheng P, Cai D, Zhao J, Shen W (2017) Thermodynamic properties of mixed surfactants of dodecyltrimethylammonium bromide and 1-dodecyl-3-methylimidazolium bromide. J Chem Thermodynamics 115:394–398. https://doi.org/10.1016/j.jct.2016.10.039
Qi X, Zhang X, Luo G, Han C, Liu C, Zhang S (2013) Mixing behaviour of conventional cationic surfactants and ionic liquid surfactant 1-tetradecyl-3-methylimidazolium bromide [C14mim][Br] in aqueous medium. J Disp Technol 34:125–133. https://doi.org/10.1080/01932691.2011.653926
Dutta R, Ghosh S, Banerjee P, Kundu S, Sarkar N (2016) Micelle-vesicle-micelle transition in aqueous solutions of anionic surfactant and cationic imidazolium surfactants: alteration of the location of different fluorophores. J Colloid Interface Sci 490:762–773. https://doi.org/10.1016/j.jcis.2016.12.009
Wang GY, Wang YY, Wang XH (2017) Aggregation behaviours of mixed systems for the imidazole based ionic liquid surfactant and triton X-100. J Mol Liq 232:55–61. https://doi.org/10.1016/j.molliq.2017.02.044
Dutta R, Kundu S, Sarkar N (2018) Ionic liquid-induced aggregate formation and their formation. Biophys Rev 10:861–871. https://doi.org/10.1007/s12551-018-0408-5
Ghosh S, Ghatak C, Banerjee C, Mandal S, Kuchlyan J, Sarkar N (2013) Spontaneous transition of micelle-vesicle-micelle in a mixture of cationic surfactant and anionic surfactant-like ionic liquid: a pure non-lipid small uni-lamellar vesicular template used for solvent and rotational relaxation study. Langmuir 29(32):10066–10076. https://doi.org/10.1021/la402053a
Thakkar K, Patel V, Ray D, Pal H, Aswal VK, Bahadur P (2016) Interaction of imidazolium based ionic liquids with triton X-100 micelles: investigating the role of the counter ion and chain length. RSC Adv 6:36314–36326. https://doi.org/10.1021/je400861g
Zhang Z, Wei Y, Wang F, Ren C, Lin Y (2014) Micellization and thermodynamic study of 1-Alkyl-3-Methylimdazolium tetra fluoroborate ionic liquids in aqueous solution. J Chem Eng Data 59(4):1120–1129. https://doi.org/10.1021/je400861g
Ali A, Farooq U, Uzair S, Patel R (2016) Conductometric and tensiometric studies on the mixed micellar systems of surface active ionic liquid and cationic surfactants in aqueous medium. J Mol Liq 223:589–602. https://doi.org/10.1016/j.molliq.2016.08.0
Aswal VK, Padsala S, Dharaiya N, Ray D, , Sastry NV, Bahadur P (2018) Self-organization of mixtures of sodium oleate and imidazolium based surface active ionic liquids studied by tensiometry, rheology and neutron scattering. J Mol Liq 249:573–582. https://doi.org/10.1016/j.molliq.2017.10.108
Sastry NV, Vaghela NM, Macwan PM, Soni SS, Aswal VK, Gibaud A (2012) Aggregation behaviour of pyridinium based ionic liquids in water- surface tension, 1H NMR chemical shifts, SANS and SAXS measurements. J Colloid Interface Sci 371:52–61. https://doi.org/10.1016/j.jcis.2011.12.077
Bandres I, Meler S, Giner B, Cea P, Lafuente C (2009) Aggregation behaviour of pyridinium based ionic liquids in aqueous solution. J Solut Chem 38:1622–1634. https://doi.org/10.1007/s10953-009-9474-4
Zhang X, Ge L, Lei Y, Liu Z, Guo R (2014) Micellization behaviour of the ionic liquid lauryl isoquinolinium bromide in aqueous solution. Colloid Polym Sci 292:1111–1120. https://doi.org/10.1007/s00396-013-3151-2
Pal A, Mann R (2018) Interactional behaviour of surface active ionic liquid lauryl isoquinolinium bromide and anionic polyelectrolyte poly(4-styrenesulfonic acid-co-maleic acid) sodium salt in aqueous solution. Colloid Polym Sci 296:483–494. https://doi.org/10.1007/s00396-018-4263-5
Clint JH (1975) Micellization of mixed non-ionic surface active agents. J Chem Soc Faraday Trans 71(1):1327–1334. https://doi.org/10.1039/F19757101327
Rubingh DN, Mittal KL (1979) Solution chemistry of surfactants, vol 337. Plenum Press, New York
Motomura K, Yamanaka M, Aratono M (1984) Thermodynamic consideration of the mixed micelle of the surfactants. Colloid Polym Sci 262:948–955. https://doi.org/10.1007/BF01490027
Aratono M, Villeneuve M, Takiue T, Ikeda N, Iyota H (1998) Thermodynamic tconsideration of mixtures of surfactants in adsorbed films and micelles. J Colloid Interface Sci 200:161–171. https://doi.org/10.1006/jcis.1997.5369
Inoue T, Ebina H, Dong B, Zheng L (2007) Electrical conductivity study on micelle formation of long-chain imidazolium ionic liquids in aqueous solution. J Colloid Interface Sci 314(1):236–241. https://doi.org/10.1016/j..jcis.2007.05.052
Singh T, Kumar A (2008) Self-aggregation of ionic liquids in aqueous media: a thermodynamic study. Colloids Surf A Physicochem Eng Asp 318(1–3):263–268. https://doi.org/10.1016/j.colsurfa.2007.12.043
Pal A, Pillania A (2015) The effect of hydrophilic ionic liquid 1-butyl-2,3-dimethylimidazolium bromide on the aggregation behaviour of tetradecyltrimethylammonium bromide in aqueous media. J Mol Liq 209:6–13. https://doi.org/10.1016/j.molliq.2015.04.0631
Junquera E, Aicart E (2002) Mixed micellization of dodecylethyldimethylammonium bromide and docecyltrimethylammonium bromide in aqueous solution. Langmuir 18(24):9250–9258. https://doi.org/10.1021/la026121p
Rosen MJ (1988) Surfactants and interfacial phenomenon2nd edn. Wiley, New York
Azum N, Naqui AZ, Akram M, Din-Ud K (2008) Mixing behaviour of conventional and gemini cationic surfactants. J Dispers Sci Technol 29:711–717. https://doi.org/10.1080/01932690701756735
Zhang S, Gao Y, Dong B, Zheng L (2010) Interaction between the added long-chain ionic liquid 1-dodecyl-3-methylimidazolium tetrafluoroborate and triton X-100 in aqueous solutions. Colloids Surf A Physicochem Eng Asp 372:182–189. https://doi.org/10.1016/j.colasurfa2010.10.011
Funding
The author acknowledge the financial support for work by the Council of Scientific and Industrial Research (CSIR), Government of India (Grant No. 21(1005)/15/EMR-II) through Emeritus Scientist grant of Prof. A. Pal.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 237 kb)
Rights and permissions
About this article
Cite this article
Pal, A., Punia, R. Interaction study of mixed micellar system of isoquinoline based surface active ionic liquids and cationic surfactant in aqueous medium. Colloid Polym Sci 297, 1011–1024 (2019). https://doi.org/10.1007/s00396-019-04519-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04519-0