Abstract
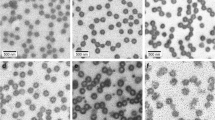
Poly(N-isopropylacrylamide) microgels prepared without exogenous cross-linker are extremely “soft” as a result of their very low cross-linking density, with network connectivity arising only from the self-crosslinking of pNIPAm chains. As a result of this extreme softness, our group and others have taken interest in using these materials in a variety of bioengineering applications, while also pursuing studies of their fundamental properties. Here, we report deswelling triggered structural changes in poly(N-isopropylacrylamide-co-acrylic acid) (ULC10AAc) microgels prepared by precipitation polymerization. Dynamic light scattering suggests that the deswelling of these particles not only depends on the collapse of the pNIPAm chains but is also influenced by the ionization state of the acrylic acid moieties present in the copolymer. The ULC10AAc microgel behaves like a traditional cross-linked pNIPAm microgel at pH 3.5, showing a sharp decrease in the hydrodynamic diameter around the lower critical solution temperature (LCST) of pNIPAm. As the pH is increased to 4.5, we observe multiple transitions in the deswelling curve, suggesting inhomogeneity in the structure and/or composition of the microgels. At pH 6.5, the microgels cease to be thermoresponsive over the studied temperature range due to increased charge repulsion between the fully deprotonated AAc groups and an increase in gel osmotic pressure due to solvated counterion ingress. Atomic force microscopy images of particles deposited at different temperatures reveal a temperature-induced morphological change, with punctate structures forming inside microgels at pH 4.5 and 6.5 and temperature above the gel volume phase transition temperature (VPTT).

.





Similar content being viewed by others
References
Kawaguchi H (2000) Functional polymer microspheres. Prog Polym Sci 25(8):1171–1210. https://doi.org/10.1016/S0079-6700(00)00024-1
Pelton R (2000) Temperature-sensitive aqueous microgels. Adv Colloid Interf Sci 85(1):1–33. https://doi.org/10.1016/S0001-8686(99)00023-8
Pelton RH, Chibante P (1986) Preparation of aqueous latexes with N-isopropylacrylamide. Colloids Surf A Physicochem Eng Asp 20(3):247–256. https://doi.org/10.1016/0166-6622(86)80274-8
Pichot C, Taniguchi T, Delair T, Elaissari A (2003) Functionalized thermosensitive latex particles: useful tools for diagnostics. J Dispers Sci Technol 24(3 & 4):423–437. https://doi.org/10.1081/DIS-120021799
Zhang H, Mardyani S, Chan WCW, Kumacheva E (2006) Design of biocompatible chitosan microgels for targeted pH-mediated intracellular release of cancer therapeutics. Biomacromolecules 7(5):1568–1572. https://doi.org/10.1021/bm050912z
Hoare T, Pelton R (2006) Titrametric characterization of pH-induced phase transitions in functionalized microgels. Langmuir 22(17):7342–7350. https://doi.org/10.1021/la0608718
Hoare T, Pelton R (2007) Engineering glucose swelling responses in poly(N-isopropylacrylamide)-based microgels. Macromolecules 40(3):670–678. https://doi.org/10.1021/ma062254w
Campbell SB, Hoare T (2014) Externally addressable hydrogel nanocomposites for biomedical applications. Curr Opin Chem Eng 4:1–10. https://doi.org/10.1016/j.coche.2013.12.003
Hoare T, Pelton R (2004) Highly pH and temperature responsive microgels functionalized with vinylacetic acid. Macromolecules 37(7):2544–2550. https://doi.org/10.1021/ma035658m
Smeets NMB, Hoare T (2013) Designing responsive microgels for drug delivery applications. J Polym Sci A Polym Chem 51(14):3027–3043. https://doi.org/10.1002/pola.26707
Nayak S, Lee H, Chmielewski J, Lyon LA (2004) Folate-mediated cell targeting and cytotoxicity using thermoresponsive microgels. J Am Chem Soc 126(33):10258–10259. https://doi.org/10.1021/ja0474143
Kim J, Singh N, Lyon LA (2006) Label-free biosensing with hydrogel microlenses. Angew Chem Int Ed 45(9):1446–1449. https://doi.org/10.1002/anie.200503102
Nolan CM, Serpe MJ, Lyon LA (2004) Thermally modulated insulin release from microgel thin films. Biomacromolecules 5(5):1940–1946. https://doi.org/10.1021/bm049750h
Serpe MJ, Yarmey KA, Nolan CM, Lyon LA (2005) Doxorubicin uptake and release from microgel thin films. Biomacromolecules 6(1):408–413. https://doi.org/10.1021/bm049455x.
Smith MH, Lyon LA (2012) Multifunctional nanogels for siRNA delivery. Acc Chem Res 45(7):985–993. https://doi.org/10.1021/ar200216f
Pelton RH, Pelton HM, Morphesis A, Rowell RL (1989) Particle sizes and electrophoretic mobilities of poly(N-isopropylacrylamide) latex. Langmuir 5(3):816–818. https://doi.org/10.1021/la00087a040
O'Reilly RK, Joralemon MJ, Hawker CJ, Wooley KL (2006) Facile syntheses of surface-functionalized micelles and shell cross-linked nanoparticles. J Polym Sci, Part A: Polym Chem 44(17):5203–5217. https://doi.org/10.1002/pola.21602
Slater M, Snauko M, Svec F, Frechet JMJ (2006) “Click chemistry” in the preparation of porous polymer-based particulate stationary phases for μ-HPLC separation of peptides and proteins. Anal Chem 78(14):4969–4975. https://doi.org/10.1021/ac060006s
Vinogradov SV, Bronich TK, Kabanov AV (2002) Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev 54(1):135–147. https://doi.org/10.1016/S0169-409X(01)00245-9
Saunders BR, Crowther HM, Vincent B (1997) Poly[(methyl methacrylate)-co-(methacrylic acid)] microgel particles: swelling control using pH, cononsolvency, and osmotic deswelling. Macromolecules 30(3):482–487. https://doi.org/10.1021/MA961277F
Islam MR, Serpe MJ (2013) Penetration of polyelectrolytes into charged poly(N-isopropylacrylamide) microgel layers confined between two surfaces. Macromolecules (Washington, DC, U S) 46(4):1599–1606. https://doi.org/10.1021/ma302637n
Hoare T, Pelton R (2004) Functional group distributions in carboxylic acid containing poly(N-isopropylacrylamide) microgels. Langmuir 20(6):2123–2133. https://doi.org/10.1021/la0351562
Gao J, Frisken BJ (2003) Cross-linker-free N-isopropylacrylamide gel nanospheres. Langmuir 19(13):5212–5216. https://doi.org/10.1021/la0269762
Gao J, Frisken BJ (2003) Influence of reaction conditions on the synthesis of self-cross-linked N-isopropylacrylamide microgels. Langmuir 19(13):5217–5222. https://doi.org/10.1021/la034207s
Brown AC, Stabenfeldt SE, Ahn B, Hannan RT, Dhada KS, Herman ES, Stefanelli V, Guzzetta N, Alexeev A, Lam WA, Lyon LA, Barker TH (2014) Ultrasoft microgels displaying emergent platelet-like behaviours. Nat Mater 13(12):1108–1114. https://doi.org/10.1038/nmat4066
Sproul EP, Nandi S, Roosa C, Schreck L, Brown AC (2018) Biomimetic microgels with controllable deformability improve healing outcomes. Adv Biosyst 2(10):1800042. https://doi.org/10.1002/adbi.201800042
Bachman H, Brown AC, Clarke KC, Dhada KS, Douglas A, Hansen CE, Herman E, Hyatt JS, Kodlekere P, Meng Z, Saxena S, Spears Jr MW, Welsch N, Lyon LA (2015) Ultrasoft, highly deformable microgels. Soft Matter 11(10):2018–2028. https://doi.org/10.1039/C5SM00047E
Gaulding JC, Spears MW, Lyon LA (2013) Plastic deformation, wrinkling, and recovery in microgel multilayers. Polym Chem 4(18):4890–4896. https://doi.org/10.1039/C3PY00173C
Welsch N, Lyon LA (2017) Oligo(ethylene glycol)-sidechain microgels prepared in absence of cross-linking agent: polymerization, characterization and variation of particle deformability. PLoS One 12(7):e0181369/1–e0181369/23. https://doi.org/10.1371/journal.pone.0181369
South AB, Whitmire RE, García AJ, Lyon LA (2009) Centrifugal deposition of microgels for the rapid assembly of nonfouling thin films. ACS Appl Mater Interfaces 1(12):2747–2754. https://doi.org/10.1021/am9005435.
Daly E, Saunders BR (2000) Temperature-dependent electrophoretic mobility and hydrodynamic radius measurements of poly(N-isopropylacrylamide) microgel particles: structural insights. Phys Chem Chem Phys 2(14):3187–3193. https://doi.org/10.1039/b002678f
Hoare T, Pelton R (2005) Electrophoresis of functionalized microgels: morphological insights. Polymer 46(4):1139–1150. https://doi.org/10.1016/j.polymer.2004.11.055
Chi W, Shuiqin Z, Au-yeung SCF, Suhong J (1996) Volume phase transition of spherical microgel particles. Die Angewandte Makromolekulare Chemie 240(1):123–136. https://doi.org/10.1002/apmc.1996.052400111
Saunders BR (2004) On the structure of poly(N-isopropylacrylamide) microgel particles. Langmuir 20(10):3925–3932. https://doi.org/10.1021/la036390v
Funding
Financial support from the National Institute of Health (R01HL130918) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Islam, M.R., Tumbarello, M. & Lyon, L.A. Deswelling induced morphological changes in dual pH- and temperature-responsive ultra-low cross-linked poly(N-isopropyl acrylamide)-co-acrylic acid microgels. Colloid Polym Sci 297, 667–676 (2019). https://doi.org/10.1007/s00396-019-04492-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-019-04492-8