Abstract
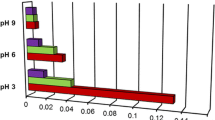
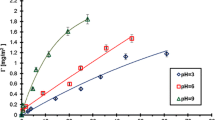
In the present study, particular emphasis was put on the effects of the type of functional groups of anionic and cationic polyacrylamide (PAM) and solution pH on the mechanism of polymer adsorption on the surface of dispersed alumina. The polymer-adsorbed amount, surface charge density and zeta potential of solid particles without and with PAM, as well as the stability of Al2O3 suspension containing the polymer, were determined. For this purpose, the spectrophotometry, potentiometric titration, microelectrophoresis and turbidimetry were applied. It was shown that adsorption of anionic PAM decreases with the pH rise, whereas in the case of cationic PAM, it increases. High adsorption level is a result of more coiled structure of adsorbed macromolecules. The anionic PAM has greater impact on the suspension stability deterioration in comparison to the cationic polymer in the whole range of studied pH values. The more pronounced effect of alumina suspension destabilization in the anionic PAM presence was obtained at pH 6, at which solid surface charge neutralization occurs.







Similar content being viewed by others
References
Grządka E, Mendrek B, Chibowski S, Wiśniewska M, Trzebicka B (2014) Investigations of the properties of the manganese dioxide suspension in the presence of guar gum and carboxymethyl cellulose. Mat Chem Phys 144:361–368
Nosal-Wiercińska A (2012) Adsorption of cystine at mercury/ aqueous solution of chlorate (VII) interface in solutions of different water activity. Cent Eur J Chem 10:1290–1300
Nosal-Wiercińska A, Grochowski M (2011) Adsorption of thiourea and its methyl derivatives from chlorate(VII) with varied water activity. Coll Czech Chem Comm 76:265–275
Nosal-Wiercińska A, Dalmata G (2010) Adsorption of methonine at merkury/aqueous solution of chlorate (VII) interface; dependence on the supporting electrolyte concentration. Elecroanal 22:2081–2086
Napper DH (1983) Polymeric stabilization of colloidal dispersions. Academic Press, INC
Yu J, Wang D, Ge X, Yan M, Yang M (2006) Flocculation of kaolin particles by two typical polyelectrolytes: a comparative study of the kinetics and floc stricture. Colloids Surf 290:288–294
Kamibayashi M, Ogura H, Otsubo Y (2008) Shear-thickening flow of nanoparticle suspension flocculated by polymer bridging. J Colloid Interf Sci 321:294–301
Proskurina VE, Myagchenkov VA (2009) Flocculation of the titanium dioxide suspension (anatase) with compositions from anionic and cationic acrylamide copolymers. J Water Chem Technol 31:156–162
Manor O, Chau TT, Stevens GW, Chan DY, Grieser F, Dagastine RR (2012) Polymeric stabilized emulsions: steric effects and deformation in soft systems. Langmuir 28:4599–4604
Hang ZJ, Shi L, Feng X, Xiao L (2009) Electrostatic and electrosteric stabilization of aqueous suspensions of barite nanoparticles. Powder Tech 192:166–170
Leong YK (1996) Depletion interaction in colloidal suspensions: a comparison between theory and experiment. Colloids Surf 118:107–114
Asakura S, Oosawa F (1958) Interaction between particles suspended in solutions of macromolecules. J Polym Sci 33:183–192
Gurumoorthy AVP, Kha KH (2011) Polymers at interfaces: biological and non-biological applications. Rec Res Sci Tech 3:80–86
Tadros TF (2008) Colloids aspects of cosmetic formulations with particular reference to polymeric surfactants. Colloids Interf Sci Ser 4:1–34
Shahidi F, Arachchi JKV, Yeon YJ (1999) Food application of chitin and chitosans. Trends Food Sci Tech 10:37–51
Sojka RE, Bjorneberg DL, Entry JA, Lentz RD, Orts WJ (2007) Polyacrylamide in agriculture and environmental land management. Adv Agronomy 92:75–162
Tripathy T, De BR (2006) Flocculation: a new way to treat the waste water. J Phys Sci 19:93–127
Jin YZ, Zhang YF, Chen XP, Gao HS (2003) Application of organic polymeric flocculants in centrifugal dewatering of oil refinery sludge. J Environ Sci 15:510–513
Haydar S, Aziz JA (2009) Coagulation-flocculation studies of tannery wastewater using combination of alum with cationic and anionic polymers. J Hazard Mat 168:1035–1040
Wiśniewska M, Chibowski S, Urban T (2014) Effect of the presence of cationic polyacrylamide on the surface properties of aqueous alumina suspension—stability mechanism. Appl Surf Sci 320:843–851
Wiśniewska M, Chibowski S, Urban T (2015) Modification of the alumina surface properties by adsorbed anionic polyacrylamide—impact of polymer hydrolysis. J Ind Eng Chem 21:925–931
Zhang XC, Miller WP (1996) Polyacrylamide effect on infiltration and erosion in furrows. Soil Sci Soc Am J 60:866–872
Pefferkorn E (1999) Polyacrylamide at solid/liquid interfaces. J Colloid Interf Sci 216:197–220
Medvedovski E (2006) Alumina-mullite ceramics for structural applications. Ceram Int 32:369–375
Gandhi RM, Viswanathan N, Meenakshi S (2010) Preparation and application of alumina/chitosan biocomposite. Int J Biol Macromol 47:146–154
Kasprzyk-Hordern B, Dąbrowsk A, Świetlik J, Nawrocki J (2004) The application of the perfluorinated bonded alumina phase for natural organic matter catalytic ozonation. J Env Engin Sci 3:41–50
Minczewski J, Marczenko Z (1985) Analytical chemistry. National Scientific Publishing House, Warsaw
Scoggins MW, Miller JW (1975) Spectrophotometric determination of water soluble organic amides. Anal Chem 47:152–154
Crummett WB, Hummel RA (1963) The determination of traces of polyacrylamides in water. J Am Water Works Assoc 1:209–219
M’Pandou A, Siffert B (1987) Polyethylene glycol adsorption at the TiO2-H2O interface: distortion of ionic structure and shear plane position. Colloids Surf 24:159–172
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, New York
Garvey MJ, Tadros TF, Vincent B (1974) A comparison of the volume occupied by macromolecules in the adsorbed state and in the bulk solution. J Colloid Interf Sci 49:57–68
Janusz W (1999) Electrical double layer at the metal oxide/electrolyte interface. Interfacial forces and fields: theory and applications surfactant science, Vol. 85, M. Dekker, New York
Kasprzyk-Hordern B (2004) Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv Colloid Interf Sci 110:19–48
Chibowski S, Paszkiewicz M (2007) Competitive adsorption of polyacrylic acid (PAA) and polyvinyl alcohol (PVA) at Al2O3/solution interface. Surfactants and Dispersed Systems in Theory and Practice, K. Wilk(ed), PALMAPress, Wroclaw, 283–286
Acknowledgments
The study was supported by the Polish National Center of Science, grant no. 2012/07/B/ST4/00534.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiśniewska, M., Chibowski, S. & Urban, T. Impact of anionic and cationic polyacrylamide on the stability of aqueous alumina suspension—comparison of adsorption mechanism. Colloid Polym Sci 293, 1171–1179 (2015). https://doi.org/10.1007/s00396-015-3509-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3509-8