Abstract
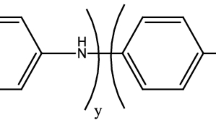
It is well accepted that the morphology of the nanomaterials has great effect on the properties and hence their applications. Therefore, morphology of materials has become a focus of research in the scientific world. The present study shows that interfacial polymerization and subsequent self-assembly provides a control over the morphology, nanorod/nanosheet, of polyaniline (PANI) films synthesized by liquid–liquid interface reaction technique and solid–liquid interface reaction technique. The synthesized PANI films and its particulate structure are characterized by using various spectroscopic techniques such as UV–visible, ATR-IR, Raman and XPS. The study confirmed the formation, the structure, the size and shape of particles and morphology of PANI by using analytical techniques namely, SAED, SEM and TEM. An important observation is that doping with HCl significantly improves the nanorod formation at the interface. The doped PANI electrode exhibits a higher area with rectangular shape in CV cycle and better cycle stability when compared with the performance of undoped PANI films. We believe that the results of these studies can give valuable leads to manoeuvre formation of PANI films with desired morphology for various applications.

Time and temperature-dependent morphology of PANI layer leading to the formation of one/two-dimensional structures namely, PANI rods/sheets, is achieved by monitoring of self-assembly of nano particulate film formed at liquid–liquid/solid–liquid interfaces









Similar content being viewed by others
References
Huang J, Kaner RB (2004) A general chemical route to polyaniline nanofibers. Journal of the American Chemical Society 126:851–855
Fan H, Wang H, Zhao N, Zhanga X, Xu J (2012) Hierarchical nanocomposite of polyaniline nanorods grown on the surface of carbon nanotube for high performance supercapicator electrode. Journal of Materials Chemistry 22:2774–2780
Stejskal J, Sapurina I, Trchova M (2010) Polyaniline nanostructures and the role of aniline oligomers in their formation. Progress in Polymer Science 35:1420–1481
Kuila BK, Nandan B, Bohme M, Janke A, Stamm M (2009) Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties. Chemical Communications 14:5749–5751
Henglein A (1993) Physicochemical properties of small metal particles in solution: microelectrode reactions, chemisorption, composite metal particles, and the atom-to-metal transition. The Journal of Physical Chemistry 97:5457–5471
Ung T, Giersig M, Dunstan D, Mulvanty P (1997) Spectroelectrochemistry of colloidal silver. Langmuir 13:1773–1782
Brus L (1996) Semiconductor colloids:individual nanocrystals, opals and porous silicon. Current Opinion in Colloid & Interface Science 2:197–201
Laura LB, Christopher KO (1997) Nanocomposite materials for optical applications. Chemistry of Materials 9:1302–1317
Virji S, Huang J, Kaner RB, Weiller BH (2004) Polyaniline nanofiber gas sensors: examination of response mechanisms. Nano Letters 4:491–496
Liu H, Kameoka J, Czaplewski DA, Craighead HG (2004) Vertically oriented arrays of polyaniline nanorods and their super electrochemical properties. Nano Letters 4:671–675
Sun S, Hoa, Ho-Si P, Harrison DJ (1991) Preparation of active Langmuir–Blodgett films of glucose oxidase. Langmuir 7:727–737
Di W, Ivaska A (2006) Electrochemical biosensors based on polyaniline. Chemist-Analyst 51:839–852
Ansari R (2006) Application of polyaniline and its composites for adsorption/recovery of chromium (VI) from aqueous solutions. Acta Chimica Slovenica 53:88–94
Amaya T, Saiom D, Hirao T (2007) Template synthesis of polyaniline/Pd nanoparticle and its catalytic application. Tetrahedron Letters 48:2729–2732
Park JE, Park SG, Koukitu A, Hatozaki O, Oyama N (2004) Effect of adding Pd nanoparticles to dimercaptan-polyaniline cathodes for lithium polymer battery. Synthetic Metals 140:121–126
Chen Z, Xu L, Li W, Waje M, Yan Y (2006) Polyaniline nanofibre supported platinum nanoelectrocatalysts for direct methanol fuel cells. Nanotechnology 17:5254
Lamy C, Belgsir EM, Le’ger JM (2004) Electrocatalytic oxidation of aliphatic alcohols: application to the direct alcohol fuel cell (DAFC). Journal of Applied Electrochemistry 31:799–809
Chiou NR, Guan J, Epstein A (2007) Growth and alignment of polyaniline nanofibres with superhydrophobic, superhydrophilic and other properties. Nature Nanotechnology 2:354–357
Liu J, Zhou M, Fan LZ, Li P, Qu X (2010) Porous polyaniline exhibits highly enhanced electrochemical capacitance performance. Electrochimica Acta 55:5819–5822
Chen Z, Qin Y, Weng D, Xiao Q, Peng Y, Wang X, Li H, Wei F, Lu Y (2009) Design and synthesis of hierarchical nanowire composites for electrochemical energy storage. Advanced Functional Materials 19:3420–3426
Inamdar AI, Kim YS, Sohn JS, Im H, Kim H, Kim DY, Kalubarme RS, Park CH (2011) Supercapacitive characteristics of electrodeposited polyaniline thin films grown on indium doped tin oxide substrates. Journal of the Korean Physical Society 59:145–149
Tseng RJ, Baker CO, Shedd B, Huang J, Kaner RB, Ouyang J, Yang Y (2007) Charge transfer effect in the polyaniline-gold nanoparticle memory system. Applied Physics Letters 90:53101
Kaynak A, Wang L, Hurren C, Wang X (2002) Characterization of conductive polypyrrole coated wool yarn. Fiber Polymer 3:24–30
Amm DT, Johnson DJ, Laursen T, Gupta SK (1992) Fabrication of ultrathin metal oxide films using Langmuir–Blodgett deposition. Applied Physics Letters 61:522–524
Sathaye SD, Patil KR, Paranjape DV (1996) process for preparation of uniform thin films of metal oxide, metal chalcogenides and metal halide. U S Patent 5, 549, 931
Sathaye SD, Patil KR, Paranjape DV, Mitra A, Awate SV, Mandale AB (2000) Preparation of Q-cadmium sulfide ultrathin films by a new liquid–liquid interface reaction technique (LLIRT). Langmuir 16:3487–3490
Petty MC, Monkman AP, Goldenberg LM (1994) A comparative study of the electrochemical properties of dip coated, spun and Langmuir–Blodgett films of polyaniline. Journal of the Electrochemical Society 141:1573–1576
Sathaye SD, Patil KR, Kulkarni SD, Bakre PP, Pradhan SD, Sarwade BD, Shintre SN (2003) Modification of spin coating method and its application to grow thin films of cobalt ferrite. Journal of Materials Science 38:29–33
Patil KR, Hwang YK, Kim MJ, Chang JS, Park SE (2004) Preparation of thin films comprising palladium nanoparticles by a solid–liquid interface reaction technique. J of Colloid and Interface Science 276:333–338
He Y (2005) Interfacial synthesis and characterization of polyaniline nanofibers. Material science and engineering B 122:76–79
Shukla SK, Bharadvaja A, Tiwari A, Parashar GK, Dubey GC (2010) Synthesis and characterization of highly crystalline polyaniline film promising for humid sensor. Adv Mat Lett 1:129–134
Ameen S, Akhtar MS, Kim YS, Yang OB, Shin HY (2010) Sulfamic acid doped polyaniline nanofibers thin film based counter electrode: application in dye sensitized solar cells. The Journal of Physical Chemistry C114:4760–4764
Zang XY, Goux WJ, Warren JC, Sanjeev KM (2004) Synthesis of polyaniline nanofibers by nanofiber seeding. Journal of the American Chemical Society 126:4502–4503
Izumi MS, Gustavo FS, Temperini LA (2008) Surface enhanced resonance Raman scattering of polyaniline on silver and gold colloids. The Journal of Physical Chemistry 112:16330–16340
Tamburri E, Orlanducci S, Guglielmotti V, Reina G, Rossi M, Terranova ML (2011) Engineering detonation nanodiamond-polyaniline composites by electrochemical routes: structural features and functional characterizations. Polymer 52:5001–5008
Nalwa H (1991) Structural determination of a semiconductive tetramer of aniline by IR, UV–visible, ESR, XPS and mass spectroscopy techniques. Journal of Materials Science 26:1683–1690
Snauwaert P, Lazzaroni R, Riga J, Verbist JJ (1987) Electronic structure of polyaniline and substituted derivatives. Synthetic Metals 18:335–340
Salaneck WR, Lundstrom I, Hjertberg T, Duke CB, Conwell E, Paton A, MacDiarmid AG, Somasiri LD, Huang WS, Richter AF (1987) Electronic structure of some polyaniline. Synthetic Metals 18:291–296
Huang JX, Kaner RB (2004) Nanofiber formation in the chemical polymerization of aniline: a mechanistic study. Angewandte Chemie, International Edition 43:5817–5821
Jnata J, Josowicz M (2003) Conducting polymers in electronic chemical sensors. Nature Materials 2:19–24
Huang J, Virji S, Weiller BH, Kaner RB (2003) Polyaniline nanofibers: facile synthesis and chemical sensor. Journal of the American Chemical Society 125:314–315
Chiou NR, Lu C, Guan J, Lee J, Epstein J (2007) Growth and alignment of polyaniline nanofibres with superhydrophobic, superhydrophilic and other properties. Nature Nanotechnology 2:354–357
Zhang X, Chan-Yu-King R, Jase A, Manohar SK (2004) Nanofibers of polyaniline synthesized by interfacial polymerization. Synthetic Metals 145:23–29
Tran HD, D’Arcy JM, Wang Y, Beltramo JP, Kaner RB (2011) The oxidation of aniline to produce polyaniline: a process yielding many different nanoscale structures. J Mate Chem 21:3534–3550
Pereira Da Silva MA, Balogh DT, Eiras C, Kleinke MU, Faria RM (2010) Analysis of polyaniline films using atomic force microscopy. Molecular Crystals and Liquid Crystals 374:191–200
Pud AA, Tabellout M, Kassibs N, Korzhenko AA, Rogalsky SP, Shapoval GS, Houze F, Schneegans O, Emery JR (2001) The poly(ethylene terephthalate)/polyaniline composite: AFM, DRS and EPR investigations of some doping effects. Journal of Materials Science 36:3355–3363
Nuraje N, Su K, Yang NL, Matsui H (2008) Liquid/liquid interfacial polymerisation to grow single crystalline nanoneedles of various conducting polymers. ACS Nano 2:502–506
Ke WJ, Lin GH, Hsu CP, Chen CM, Cheng YS, Jen TH, Chen SA (2011) Solution processable self doped polyaniline as hole transport layer for inverted polymer soler cells. Journal of Materials Chemistry 21:13483–13489
Chiou NR, Epestein AJ (2005) A simple approach to control the growth of polyaniline nanofibers. Synthetic Metals 153:69–72
Huang JX, Kaner RB (2004) Flash welding of conducting polymer nanofibres. Nature Materials 3:783–786
Zhang X, Chechik V, Smith DK, Walton PH, Duhme-Klair AK (2008) Controlled synthesis of optically active polyaniline nanorods and nanostructured gold microspheres using tetrachloroaurate as an efficient oxidant of aniline. Macromolecules 41:3417–3421
Chen Y, Yang G, Zhang Z, Yang X, Hou W, Zhu J (2010) Polyaniline intercalated layered vanadium oxide nanocomposites—one pot hydrothermal synthesis and application in lithium battery. Nanoscale 2:2131–2138
Singu BS, Srinivasan P, Srinivas P (2012) Benzoyl peroxide oxidation route to nano form polyaniline salt containing dual dopants for pseudocapacitor batteries and energy storage. J Electrochem Society 159:A6–A13
Sapurina I, Riede A, Stejskal J (2001) In-situ polymerized polyaniline films: film formation. Synthetic Metals 123:503–507
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 9.50 mb)
Rights and permissions
About this article
Cite this article
Waghmode, B.J., Patil, S.H., Jahagirdar, M.M. et al. Studies on morphology of polyaniline films formed at liquid–liquid and solid–liquid interfaces at 25 and 5 °C, respectively, and effect of doping. Colloid Polym Sci 292, 1079–1089 (2014). https://doi.org/10.1007/s00396-013-3150-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-013-3150-3