Abstract
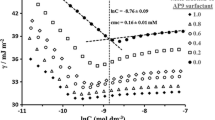
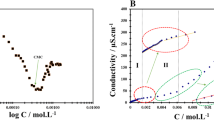
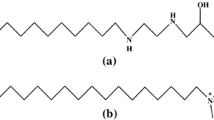
Phenomenon of clouding in charged micellar solutions is a fairly recent addition to conventional phenomenon shown by aqueous nonionic micelles. In this paper, we have tested a Hofmeister-like ordering of charged headgroups in the context of cloud point (CP) and micellar growth. For this purpose, we have used various combinations of surfactant (sodium dodecyl sulfate, SDS; sodium dodecylbenzene sulfonate, SDBS; sodium salts of α-sulfonato myristic acid methyl ester, MES; and α-sulfonato palmitic acid methyl ester, PES) and tetra-n-butylammonium bromide (TBAB). Different surfactant concentrations and TBAB concentrations are used and CP measurements have been performed. CP values were found in the order SDBS < SDS < PES < MES for the same concentration of surfactant and TBAB. This order has been discussed in the light of water affinities of interacting ionic species (i.e., surfactant headgroup and TBA+ counterion). The ordering was found similar for the case of micellar growth studied by dynamic light scattering (DLS). A bimodal distribution of aggregate size was found that transforms to giant aggregates at CP. The micelles of roughly 10-nm size convert to aggregates of 1 μm. The study has a few novelties: (1) headgroup dependence of CP, (2) micellar growth on heating, and (3) confirmation of Hofmeister-like series of headgroup.





Similar content being viewed by others
References
Mukherjee P, Padhan SK, Dash S, Patel S, Mishra BK (2011) Clouding behavior in surfactant systems. Adv Colloid Interface Sci 162:59–79. doi:10.1016/j.cis.2010.12.005
Zhang J, Li J, Zhao Y, Han B, Hou M, Yang G (2011) Efficient separation of surfactant and organic solvent by CO2. Chem Comm 47:5816–5818. doi:10.1039/c0cc05768A
Sreejith L, Parathakkat S, Nair SM, Kumar S, Varma G, Hassan PA, Talmon Y (2011) Octanol triggered self assemblies of the CTAB/KBr system: a microstructural study. J Phys Chem B 115:464–470. doi:10.1021/jp1043255
Kumar S, Sharma D, Din Kabir-ud (2000) Cloud point phenomenon in anionic surfactant + quaternary bromide systems and its variation with additives. Langmuir 16:6821–6824. doi:10.1021/la9913607
Warr GG, Zemb TN, Drifford M (1990) Liquid–liquid phase separation in cationic micellar solution. J Phys Chem 94:3086–3092. doi:10.1021/j100370a063
Bales BL, Zana R (2004) Cloud point of aqueous solutions of tetrabutylammonium dodecyl sulfate is a function of the concentration of counter ions in the aqueous phase. Langmuir 20:1579–1581. doi:10.1021/la0353935
Kumar S, Bhadoria A (2012) Thermodynamic energetic of charged micellar solutions with and without salt at the cloud point. J Chem Eng Data 57:521. doi:10.1021/je200909j
Ahmad T, Kumar S, Khan ZA, Din Kabir-ud (2007) Additives as cp modifiers in an anionic micellar solution. Colloids Surf A 294:130–136. doi:10.1016/j.colsurfa.2006.08.003
Kumar S, Bhadoria A, Patel H, Aswal VK (2012) Morphologies near cloud point in aqueous ionic surfactant: scattering and NMR studies. J Phys Chem B 116:3699–3703. doi:10.1021/jp300630w
Kumar S, Sharma D, Din Kabir-ud (2003) Temperature–[salt] compensation for clouding in ionic micellar systems containing sodium dodecyl sulfate and symmetrical quaternary bromides. Langmuir 19:3539–3541. doi:10.1021/la026783e
Mitra D, Chakraborty I, Bhattacharya SC, Moulik SP (2007) Interfacial and solution properties of tetraalkylammonium bromides and their sodium dodecyl sulfate interacted products: a detailed physicochemical study. Langmuir 23:3049–3061. doi:10.1021/la062830h
Rout DK, Chauhan S, Agarwal A (2009) Cloud point and microemulsion phase behavior of sodium linear alkylbenzene sulfonate with tetrabutyl and benzyltributyl substituted ammonium halides. Ind Eng Chem Res 48:8842–8847. doi:10.1021/ie801873f
Kunz W, Henle J, Ninham BW (2004) About the science of the effect of salts: Franz Hofmeister's historical papers. Curr Op Colloid Interf Sci 9:19–37. doi:10.1016/j.cocis.2004.05.005
Vlachy N, Jagoda-Cwiklik B, Vacha R, Touraud D, Jungwirth P, Kunz W (2009) Hofmeister series and specific interactions of charged head groups with aqueous ions. Adv Colloid Interface Sci 146:42–47. doi:10.1016/j.cis.2008.09.010
Collins KD, Neilson GW, Enderby JE (2007) Ions in water: characterizing the forces that control chemical process and biological structure. Biophys Chem 128:95–104. doi:10:1016/j.bpc.2007.03.009
Raghavan SR, Edlund H, Kaler EW (2002) Clouding phenomenon in wormlike micellar system containing cationic surfactant and salt. Langmuir 18:1056–1064. doi:10.1021/la011148e
Sein A, Engberts JBNF (1995) Micellar to lamellar aggregate transition of an anionic surfactant in dilute aqueous solution induced by alkali metal chloride and tetraalkylammonium chloride salts. Langmuir 11:455–465. doi:10.1021/a00002a015
Kabir-ud-din KS, Parveen N (2008) The clouding phenomenon for anionic sodium dodecyl sulfate + quaternary bromides in polar nonaqueous-water-mixed solvents. J Surf Deterg 11:335–341. doi:10.1007/s11743-008-1087-1
Kumar S, Parveen N, Din Kabir-ud (2004) Effect of urea addition on micellization and the related phenomenon. J Phys Chem B 104:9588–9592. doi:10.1021/jp036552w
Patil SR, Mukaiyama T, Rakshit AK (2004) α-Sulfonato palmitic acid methylester-hexaoxyethylene monododecyl ether mixed surfactant system: interfacial, thermodynamic and performance property study. J Surf Deterg 7:87–96. doi:10.1007/s11743-004-0293-y
Jansson M, Eriksson L, Skagerlind P (1991) The effect of tetraalkylammonium ions on the stability of dilute O/W emulsions. Colloids Surf 53:157–167. doi:10.1016/0166-6622(91)800042-M
Chandler D (2002) Hydrophobicity: “two faces of water”. Nature 417:491. doi:10.1038/417491a
Kumar S, Ziya AK, Din Kabir-ud (2004) Clouding phenomenon in ionic micellar solutions: role of the counterion. J Surf Deterg 7:367–371. doi:10.1007/s11743-004-0320-z
Jana R, Schmidt J, Talmon Y (2005) Tetrabutyl ammonium alkyl carboxylate surfactants in aqueous solution: self-association behavior, solution nanostructutre and comparison with tetrabutylammonium alkyl sulfate Surfactants. Langmuir 21:11628–11636. doi:10.1021/a051665n
Alam MS, Kumar S, Naqvi AZ, Din Kabir-ud (2006) Study of the cloud point of an amphiphilic antidepressent drug: influence of surfactants, polymers and non-electrolytes. Colloids Surf A 287:197–202. doi:10.1016/j.col surfa.2006.04.001
Ballesteros-Gomez A, Sicilia MD, Rubio S (2010) Supramolecular solvents in the extraction of organic compounds: a review. Anal Chimica Acta 677:108–130. doi:10.1016/j.aca.2010.07.027
Yazdi AS (2011) Surfactant based extraction methods. Trends Anal Chem 30:918–929. doi:10.1016/j.trac.2011.02.010
Casero I, Sicilia D, Rubio S, Perez-Bendito D (1999) An acid-induced phase separation approach using anionic surfactants for the extraction and preconcentration of organic compounds. Anal Chem 71:4519–4526. doi:10.1021/ac9901069
Jansson M, Jonsson A, Li P, Stilb P (1991) Aggregation in tetraalkylammonium dodecanoate systems. Colloids Surf 59:387–397. doi:10.1016/0166-6622(91)80261-L
Kalur GC, Raghavan SR (2005) Anionic wormlike micelles fluids that display cloud point: rheology and phase behavior. J Phys Chem B 109:8599–8604. doi:10.1021/la04oo33i
Jana R (2004) Partial phase behavior and micellar properties of tetrabutylammonium salts of fatty acids: unusual solubility in water and formation of unexpectedly small micelles. Langmuir 20:5666–5668. doi:10.1021/jp044102d
Desiraju GR (1995) Supramolecular synthon in crystal engineering: a new organic synthesis. Angew Chem Int Ed Eng 34:2311–2327. doi:10.1002/anie.199523111
Stubenrauch C, Classon PM, Rutland M, Manev E, Johansson I, Pedersen JS, Langevin D, Blunk D, Bain CD (2010) Mixtures of n-dodecyl-β-d-maltoside and hexaoxyethylene dodecyl ether—surface properties, bulk properties, foam films and foams. Adv Colloid Interface Sci 155:5–18. doi:10.1016/j.cis.2009.12.002
Kumar S, David SL, Aswal VK, Goyal PS, Din Kabir-ud (1997) Growth of sodium dodecyl sulfate micelles in aqueous ammonium salts. Langmuir 13:6461–6464. doi:10.1021/la970538r
Kumar S, Aswal VK, Goyal PS, Din Kabir-ud (1998) Micellar growth in the presence of quaternary ammonium salts: a SANS study. J Chem Soc Faraday Trans 94:761–764. doi:10.1039/A707590A
Han Y, Feng Y, Sun H, Li Z, Han Y, Wang H (2011) Wormlike micelles formed by sodium erucate in the presence of a tetraalkylammonium hydrotrope. J Phys Chem B 115:6893–6902. doi:10.1021/jp2004634
Yu Z-J, Xu G (1989) Physicochemical properties of aqueous mixtures of tetrabutylammonium bromide and anionic surfactants. 1. Temperature-induced micellar growth and cloud point phenomenon. J Phys Chem 93:7441–7445. doi:10.1021/j100358a037
Israelachvili JN, Mitchell DJ, Ninham BW (1976) Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J Chem Soc Faraday Trans 272:1525–1568. doi:10.1039/F29767201525
Raghavan SR, Kaler EW (2001) Highly viscoelastic wormlike micellar solutions formed by cationic surfactants with long unsaturated tails. Langmuir 17:300–306. doi:10.1021/la0007933
Vlachy N, Renoncourt A, Drechsler M, Verbavatz JM, Touraud D, Kunz W (2008) Blastulae aggregates :new intermediate structures in the micelle-to-vesicle transition of catanionic systems. J Colloid Interface Sci 320:360–363. doi:10.1016/j.jcis.2007.12.034
Porte G (1983) Giant micelle in ideal solutions. Either rods or vesicles. J Phys Chem 87:3541–3550. doi:10.1021/j 100241a036
Drecher MR, Simnick AJ, Fischer K, Smith RJ, Patel A, Schmidt M, Chilkoli A (2008) Temperature triggered self assembly of polypeptides into multivalent spherical micelles. J Am Chem Soc 130:687–694. doi:10.1021/ja0764862
Materna K, Goralska E, Sobczynska A, Szymanowski J (2004) Recovery of various phenols and phenyl amines by micellar enhanced ultra filtration and cloud point separation. Green Chem 6:176–182. doi:10.1039/B312343J
Acknowledgments
The authors are thankful to Prof. B.V. Kamath, Head, Department of Chemistry for fruitful discussions and research facilities. H. Patel is also thankful to DST, New Delhi, India for providing INSPIRE fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, S., Patel, H. & Patil, S.R. Test of Hofmeister-like series of anionic headgroups: clouding and micellar growth. Colloid Polym Sci 291, 2069–2077 (2013). https://doi.org/10.1007/s00396-013-2942-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-013-2942-9