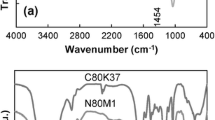
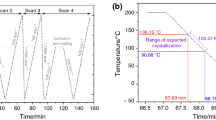
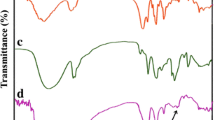
Abstract
The coil-to-globule transition of poly(N-isopropylacrylamide) (PNIPA) prepared by free-radical redox polymerization in aqueous solutions and its nanocomposite (NC) gels were investigated by differential scanning calorimetery. The lower critical solution temperatures (LCST) of aqueous solutions of PNIPA of different molecular weights were not significantly affected by molecular weight (M w: 0.19 × 106−4.29 × 106 g × mol−1) or polymer concentration (1−10 wt%), although the enthalpy of transition increased with molecular weight, at M w (<1.2 × 106 g × mol−1). The glass-transition temperature of PNIPA in the dried state also remained constant (138 °C), regardless of molecular weight. On the other hand, the enthalpy of the coil-to-globule transition of PNIPA in NC gels consisting of a PNIPA/clay network decreased with increasing clay concentration (C clay), while the onset temperature (≡LCST) was almost constant, regardless of C clay. The PNIPA chains in NC gels could be classified into the following three types: P-1, which exhibits a normal LCST transition, similar to that of linear PNIPA; P-2, exhibiting restricted transition at higher temperatures as a result of interactions with the clay; and P-3, which does not undergo that transition because of stronger restrictions. It was found that the proportion of P-3 increases with increasing C clay. However, some P-1 and P-2 was still observed, even in NC gels with high C clay. That the transition to the hydrophobic globular state was restricted by interactions with the clay was confirmed by measurements on PNIPA after removal of the clay from NC gels.







Similar content being viewed by others
Notes
There is some ambiguity about the molecular weight of PNIPA prepared in aqueous solution. In Otake’s paper [36], PNIPA was prepared using the following conditions: Free-radical redox polymerization at 0 °C, [NIPA] = 0.7 M, KPS = 0.15 mM, TEMED = 2 mL for 1 l H2O. Based on our results [34], the conditions used there should result in a partially self-crosslinked gel, the molecular weight of which cannot be precisely determined by GPC
References
Schmaljohann D (2006) Thermo-and pH-responsive polymers in drug delivery. Adv Drug Delivery Rev 58:1655–1670
Aoki T, Kawashima M, Katono H, Sanui K, Ogata N, Okano T, Sakurai Y (1994) Temperature-responsive interpenetrating polymer networks constructed with poly(acrylic acid) and poly(N, N-dimethylacrylamide). Macromolecules 27:947–952
Schild HG (1992) Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci 17:163–249
Alarcon AH, Pennadam S, Alexander C (2005) Stimuli responsive polymers for biomedical applications. Chem Soc Rev 34:276–285
Cao Z, Ziener U, Landfester K (2010) Synthesis of narrowly size-distributed thermosensitive poly(N-isopropylacrylamide) nanocapsules in inverse miniemulsion. Macromolecules 43:6353–6360
Satokawa Y, Shikata T, Tanaka F, Qiu X, Winnik FM (2009) Hydration and dynamic behavior of a cyclic poly(N-isopropylacrylamide) in aqueous solution: effects of the polymer chain topology. Macromolecules 42:1400–1403
Hu Y, Fujiwara H, Sasaki K, Tsujii K (2005) Rapid swelling/collapsing behavior of thermoresponsive poly(N-isopropylacrylamide) gel containing poly(2-(methacryloyloxy)decyl phosphate) surfactant. Angew Chem Int Ed 44:1951–1954
Kikuchi A, Okano T (2002) Pulsatile drug release control using hydrogels. Adv Drug Deliver Rev 54:53–77
Tsuji S, Kawaguchi H (2006) Effect of graft chain length and structure design on temperature-sensitive hairy particles. Macromolecules 39:4338–4344
Akashi R, Tsutsui H, Komura A (2002) Polymer gel light-modulation materials imitating pigment cells. Adv Mater 14:1808–1811
Haraguchi K, Takehisa T (2002) Nanocomposite hydrogels: a unique organic-inorganic network structure with extraordinary mechanical, optical, and swelling/de-swelling properties. Adv Mater 14:1120–1124
Haraguchi K, Takehisa T, Fan S (2002) Effects of clay content on the properties of nanocomposite hydrogels composed of poly(N-isopropylacrylamide) and clay. Macromolecules 35:10162–10171
Haraguchi K, Farnworth R, Ohbayashi A, Takehisa T (2003) Compositional effect on mechanical properties of nanocomposite hydrogels composed of poly(N, N-dimethylacrylamide) and clay. Macromolecules 36:5732–5741
Haraguchi K, Li HJ (2006) Mechanical properties and structure of polymer-clay nanocomposite gels with high clay content. Macromolecules 39:1898–1905
Haraguchi K (2007) Nanocomposite hydrogels. Curr Opin Solid State Mat Sci 11:47–54
Haraguchi K (2011) Synthesis and properties of soft nanocomposite materials with novel organic/inorganic network structures. Polym J 43:223–241
Haraguchi K, Li HJ (2005) Control of the coil-to-globule transition and ultrahigh mechanical properties of PNIPA in nanocomposite hydrogels. Angew Chem Int Ed 44:6500–6504
Haraguchi K, Li HJ, Song L, Murata K (2007) Tunable optical and swelling/deswelling properties associated with control of the coil-to-globule transition of poly(N-isopropylacrylamide) in polymer-clay nanocomposite gels. Macromolecules 40:6973–6980
Haraguchi K, Takehisa T, Ebato M (2006) Control of cell cultivation and cell sheet detachment on the surface of polymer/clay nanocomposite hydrogels. Biomacromolecules 7:3267–3275
Haraguchi K, Takada T (2010) Synthesis characteristics of nanocomposite gels prepared by in situ photopolymerization in an aqueous system. Macromolecules 43:4294–4299
Scarpa JS, Mueller DD, Klotz IM (1967) Slow hydrogen-deuterium exchange in a non-α-helical polyamide. J Am Chem Soc 89:6024–6030
Heskins M, Guillet JE (1968) Solution properties of poly(N-isopropylacrylamide). J Macromol Sci Chem 2:1441–1455
Fujishige S, Kubota K, Ando I (1989) Phase transition of aqueous solution of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide). J Phys Chem 93:3311–3313
Schild HG, Tirrell DA (1990) Microcalorimetric detection of lower critical solution temperature in aqueous polymer solution. J Phys Chem 94:4352–4356
Tiktopulo EI, Uversky VN, Lushchik VB, Klenin SI, Bychkova VE, Ptitsyn OB (1995) “Domain” coil-globule transition in homopolymers. Macromolecules 28:7519–7524
Tong Z, Zeng F, Zheng X, Sato T (1999) Inverse molecular weight dependence of cloud points for aqueous poly(N-isopropylacrylamide) solutions. Macromolecules 32:4488–4490
Furyk S, Zhang Y, Ortiz-Acosta D, Cremer PS, Bergbreiter DE (2006) Effects of end group polarity and molecular weight on the lower critical solution temperature of poly(N-isopropylacrylamide). J Polym Sci Part A 44:1492–1501
Ray B, Okamoto Y, Kamigaito M, Sawamoto M, Seno K, Kanaoka S, Aoshima S (2005) Effect of tacticity of poly(N-isopropylacrylamide) on the phase separation temperature of its aqueous solutions. Polym J 37:234–237
Kawaguchi T, Kojima Y, Osa M, Yoshizaki T (2008) Cloud points in aqueous poly(N-isopropylacrylamide) solutions. Polym J 40:455–459
Zhang Y, Furyk S, Sagle LB, Cho Y, Bergbreiter DE, Cremer PS (2007) Effects of hofmeister anions on the LCST of PNIPAM as a function of molecular weight. J Phys Chem C 111:8916–8924
Meewes M, Ricka J, De Silva M, Nyffenegger R, Binkert T (1991) Coil-globule transition of poly(N-isopropylacrylamide): a study of surfactant effects by light scattering. Macromolecules 24:5811–5816
Boutris C, Chatzi EG, Kiparissides C (1997) Characterization of the LCST behaviour of aqueous poly(N-isopropylacrylamide) solutions by thermal and cloud point techniques. Polymer 38:2567–2570
Shibayama M, Suetoh Y, Nomura S (1996) Structure relaxation of hydrophobically aggregated poly(N-isopropylacrylamide) in water. Macromolecules 29:6966–6968
Xu Y, Li G, Haraguchi K (2010) Gel formation and molecular characteristics of poly(N-isopropylacrylamide) prepared by free-radical redox polymerization in aqueous solution. Macromol Chem Phys 211:977–987
Haraguchi K, Xu Y, Li G (2010) Molecular characteristics of poly(N-isopropylacrylamide) separated from nanocomposite gels by removal of clay from the polymer/clay network. Macromol Rapid Commun 31:718–772
Otake K, Inomata H, Konno M, Saito S (1990) Thermal analysis of the volume phase transition with N-isopropylacrylamide gels. Macromolecules 23:283–289
Miyazaki S, Endo H, Karino T, Haraguchi K, Shibayama M (2007) Gelation mechanism of poly(N-isopropylacrylamide)-clay nanocomposite gels. Macromolecules 40:4287–4295
Fox TG, Flory PJ (1950) Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phy 21:581–591
Fox TG, Loshaek S (1955) Influence of molecular weight and degree of crosslinking on the specific volume and glass temperature of polymers. J Polym Sci XV:371-390
Pezzin G, Zilio-Grandi F, Sanmartin P (1970) The dependence of the glass transition temperature on molecular weight for polyvinylchloride. Eur Polym J 6:1053–1061
O’Driscoll K, Sanayei RA (1991) Chain-length dependence of the glass transition temperature. Macromolecules 24:4479–4480
Cowie JMG, Toporowski PM (1968) The dependence of glass temperature on molecular weight for poly α-methyl styrene. Eur Polym J 4:621–625
Clarson SJ, Semlyen JA, Dodgson K (1991) Cyclic polysiloxanes: 4. Glass transition temperature of poly(phenylmethylsiloxanes). Polymer 32:2823–2827
Faucher JA, Koleske JV, Santee ER, Stratta JJ, Wilson CW (1966) Glass transition of ethylene oxide polymers. J Appl Phy 37:3962–3964
Johari GP, Hallbrucher A, Mayer E (1988) Calorimetric relaxation and glass transition in poly(propylene glycols) and its monomer. J Polym Sci Part B 26:1923–1930
Faucher JA (1965) The dependence of glass transition temperature on molecular weight for poly(propylene oxide) and poly(butylene oxide). Polym Lett 3:143–145
Lewis OG (1968) Physical constants of linear homopolymers. Springer, Berlin
Smidsrфd O, Guillet JE (1969) Study on polymer-solution interactions by gas chromatography. Macromolecules 2:272–277
Acknowledgment
This work was supported by the Ministry of Education, Science, Sports and Culture of Japan (grant-in-aid 23350117).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haraguchi, K., Xu, Y. Thermal analyses of poly(N-isopropylacrylamide) in aqueous solutions and in nanocomposite gels. Colloid Polym Sci 290, 1627–1636 (2012). https://doi.org/10.1007/s00396-012-2694-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-012-2694-y