Abstract
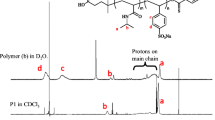
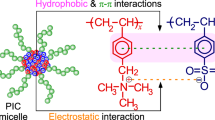
The micelle formation of poly[(4-pyridinemethoxymethyl)styrene]-block-polystyrene (PPySt-b-PSt) was studied in the nonselective solvent using perfluoroalkyl carboxylic acids. PPySt-b-PSt showed no self-assembly into micelles in THF, because this solvent was nonselective for the copolymer. Dynamic light scattering demonstrated that the diblock copolymer formed the micelles in the solvent in the presence of perfluoroalkyl carboxylic acids in which the number of carbons in the perfluoroalkyl chains was over eight. 1H NMR revealed that the micellization proceeded through the salt formation of the pyridinium perfluoroalkyl carboxylate and through the aggregation of the perfluoroalkyl chains in the counter anions. The hydrodynamic radius and the aggregation number of the micelles increased with an increase in the length of the perfluoroalkyl chain. The copolymer needed less carboxylic acid with longer perfluoroalkyl chain to form the micelles. The copolymer produced the micelles with lower aggregation number and higher critical micelle concentration at higher temperature, although the micellar size was almost independent of the temperature. The micelles were unstable with respect to the variation in the temperature, and were dissociated into the unimers with the increase in the temperature. The micelles, however, were reconstructed by decreasing the temperature. This dissociation–reconstruction of the micelles was controlled reversibly not only by the temperature but also by the concentration of the perfluoroalkyl carboxylic acid. An increase in the acid concentration suppressed the dissociation into the unimers, while promoting the reconstruction of the micelles.










Similar content being viewed by others
References
Neradovic D, Nostrum CF, Hennink WE (2001) Macromolecules 34:7589
Arotcarena M, Heise B, Ishaya S, Laschewsky A (2002) J Am Chem Soc 124:3787
Lowe AB, Billingham NC, Armes SP (1997) Chem Commun 1035
Weaver JVM, Armes SP, Butun V (2002) Chem Commun 2122
McClain JB, Canelas DA, Samulski ET, DeSimone JM, Londono JD, Cochran HD, Wignall GD, Chillura-Martino GD, Triolo R (1996) Science 274:2049
Buhler E, Dobrynin AV, DeSimone JM, Rubinstein M (1998) Macromolecules 31:7347
Zhou S, Chu B (1998) Macromolecules 31:5300
Celso L, Triolo A, Triolo F, Donato DI, Steinhart M, Kriechbaum M, Amenitsch H, Triolo R (2002) Eur Phys J (2002) Soft Matter 8:311
Koga T, Zhou S, Chu B (2001) Appl Opt 40:4170
Lee AS, Butun V, Vamvakaki M, Armes S, Pople JA, Gast AP (2002) Macromolecules 35:8540
Liu S, Weaver JVM, Tang Y, Billingham NC, Armes SP (2002) Macromolecules 35:6121
Hu Y, Kramer MC, Boudreaux CJ, McCormick CL (1995) Macromolecules 28:7100
Chernyshov DM, Bronstein LM, Boerner H, Berton B, Antonietti M (2000) Chem Mater 12:114
Liu S, Zhu H, Zha H, Jiang M, Wu C (2000) Langmuir 16:3712
Harada A, Kataoka K (1995) Macromolecules 28:5294
Martin TJ, Prochazka K, Munk P, Webber SE (1996) Macromolecules 29:6071
Gohy J-F, Lohmeijer GG, Varshney SK, Decamps B, Leroy E, Boileau S, Schubert US (2002) Macromolecules 35:9748
Liu S, Zhang G, Jiang M (1999) Polymer 40:5449
Gohy J-F, Varshney SK, Jerome R (2001) Macromolecules 34:3361
Brontein LM, Sidorov SN, Valetsky PM (1999) Langmuir 15:6256
Zhao H, Douglas EP (2002) Mater Res Soc Symp Proc 43
Miyazawa T, Endo T, Shiihashi S, Okawara M (1985) J Org Chem 50:1332
Morrison ID, Grabowski EF, Herb CA (1985) Langmuir 1:496
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshida, E., Tanaka, M. & Takata, T. Self-assembly control of a pyridine-containing diblock copolymer by perfluorinated counter anions during salt-induced micellization. Colloid Polym Sci 283, 1100–1107 (2005). https://doi.org/10.1007/s00396-004-1260-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1260-7