Abstract
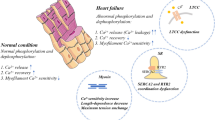
Many cardiac proteins undergo reversible phosphorylation. While the protein kinases which bring about phosphorylations are well studied, less effort has been put into the dephosphorylating phosphatases (for an earlier review compare 14). An important event in the heart, which is controlled by phosphorylation, is the uptake of Ca2+ by the sarcoplasmic reticulum (SR). This process is brought about by a SR Ca2+ ATPase (SERCA) and accounts for relaxation. The amount of Ca2+ pumped by SERCA is enhanced when phospholamban (PLB), an intrinsic protein of the SR, is phosphorylated and is diminished when PLB is dephosphorylated. PLB is dephosphorylated by protein phosphatases (PPs) like PP1. As the activity of PP1 is enhanced in heart failure, subsequent dephosphorylation by of, e.g., PLB may explain the impaired relaxation of the human heart. Thus, PPs may play an important role in the etiology and/or symptoms of heart failure.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neumann, J. Altered phosphatase activity in heart failure, influence on Ca2+ movement. Basic Res Cardiol 97 (Suppl 1), I91–I95 (2002). https://doi.org/10.1007/s003950200036
Issue Date:
DOI: https://doi.org/10.1007/s003950200036