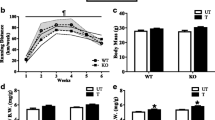
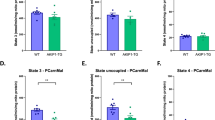
Abstract
Exercise training is key to healthful longevity. Since exercise training compliance is difficult, it would be useful to have a therapeutic substitute that mimicked exercise training. We compared the effects of exercise training in wild-type (WT) littermates with adenylyl cyclase type 5 knock out (AC5 KO) mice, a model of enhanced exercise performance. Exercise performance, measured by maximal distance and work to exhaustion, was increased in exercise-trained WT to levels already attained in untrained AC5 KO. Exercise training in AC5 KO further enhanced their exercise performance. The key difference in untrained AC5 KO and exercise-trained WT was the β-adrenergic receptor signaling, which was decreased in untrained AC5 KO compared to untrained WT but was increased in WT with exercise training. Despite this key difference, untrained AC5 KO and exercise-trained WT mice shared similar gene expression, determined by deep sequencing, in their gastrocnemius muscle with 183 genes commonly up or down-regulated, mainly involving muscle contraction, metabolism and mitochondrial function. The SIRT1/PGC-1α pathway partially mediated the enhanced exercise in both AC5 KO and exercise-trained WT mice, as reflected in the reduced exercise responses after administering a SIRT1 inhibitor, but did not abolish the enhanced exercise performance in the AC5 KO compared to untrained WT. Increasing oxidative stress with paraquat attenuated exercise performance more in untrained WT than untrained AC5 KO, reflecting the augmented oxidative stress protection in AC5 KO. Blocking nitric oxide actually reduced the enhanced exercise performance in untrained AC5 KO and trained WT to levels below untrained WT, demonstrating the importance of this mechanism. These results suggest that AC5 KO mice, without exercise training, share similar mechanisms responsible for enhanced exercise capacity with chronic exercise training, most importantly increased nitric oxide, and demonstrate more reserve with the addition of exercise training. A novel feature of the enhanced exercise performance in untrained AC5 KO mice is their decreased sympathetic tone, which is also beneficial to patients with cardiovascular disease.







Similar content being viewed by others
References
Beloka S, Gujic M, Deboeck G, Niset G, Ciarka A, Argacha JF, Adamopoulos D, Van de Borne P, Naeije R (2008) Beta-adrenergic blockade and metabo-chemoreflex contributions to exercise capacity. Med Sci Sports Exerc 40:1932–1938. doi:10.1249/MSS.0b013e31817fbe11
Braga VA, Couto GK, Lazzarin MC, Rossoni LV, Medeiros A (2015) Aerobic exercise training prevents the onset of endothelial dysfunction via increased nitric oxide bioavailability and reduced reactive oxygen species in an experimental model of menopause. PLoS ONE 10:e0125388. doi:10.1371/journal.pone.0125388
Buckenmeyer PJ, Goldfarb AH, Partilla JS, Pineyro MA, Dax EM (1990) Endurance training, not acute exercise, differentially alters beta-receptors and cyclase in skeletal fiber types. Am J of Physiol 258:E71–E77
Chen X, An X, Chen D, Ye M, Shen W, Han W, Zhang Y, Gao P (2016) Chronic exercise training improved aortic endothelial and mitochondrial function via an AMPKalpha2-dependent manner. Front Physiol 7:631. doi:10.3389/fphys.2016.00631
Davidson SR, Burnett M, Hoffman-Goetz L (2006) Training effects in mice after long-term voluntary exercise. Med Sci Sports Exerc 38:250–255. doi:10.1249/01.mss.0000183179.86594.4f
De Palma C, Morisi F, Pambianco S, Assi E, Touvier T, Russo S, Perrotta C, Romanello V, Carnio S, Cappello V, Pellegrino P, Moscheni C, Bassi MT, Sandri M, Cervia D, Clementi E (2014) Deficient nitric oxide signalling impairs skeletal muscle growth and performance: involvement of mitochondrial dysregulation. Skelet Muscle 4:22. doi:10.1186/s13395-014-0022-6
Dohm GL, Pennington SN, Barakat H (1976) Effect of exercise training on adenyl cyclase and phosphodiesterase in skeletal muscle, heart, and liver. Biochem Med 16:138–142
Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA (2009) Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol 587:3271–3285. doi:10.1113/jphysiol.2009.169771
Esposito G, Perrino C, Ozaki T, Takaoka H, Defer N, Petretta MP, De Angelis MC, Mao L, Hanoune J, Rockman HA, Chiariello M (2008) Increased myocardial contractility and enhanced exercise function in transgenic mice overexpressing either adenylyl cyclase 5 or 8. Basic Res Cardiol 103:22–30. doi:10.1007/s00395-007-0688-6
Ferrara N, Rinaldi B, Corbi G, Conti V, Stiuso P, Boccuti S, Rengo G, Rossi F, Filippelli A (2008) Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res 11:139–150. doi:10.1089/rej.2007.0576
Huang CC, Wang T, Tung YT, Lin WT (2016) Effect of exercise training on skeletal muscle SIRT1 and PGC-1alpha expression levels in rats of different age. Int J Med Sci 13:260–270. doi:10.7150/ijms.14586
Ito D, Ito O, Mori N, Cao P, Suda C, Muroya Y, Hao K, Shimokawa H, Kohzuki M (2013) Exercise training upregulates nitric oxide synthases in the kidney of rats with chronic heart failure. Clin Exp Pharmacol Physiol 40:617–625. doi:10.1111/1440-1681.12130
Ji LL, Lennon DL, Kochan RG, Nagle FJ, Lardy HA (1986) Enzymatic adaptation to physical training under beta-blockade in the rat. Evidence of a beta 2-adrenergic mechanism in skeletal muscle. J Clin Invest 78:771–778. doi:10.1172/JCI112639
Khaledi N, Fayazmilani R, Gaeini AA, Javeri A (2016) Progressive resistance training modulates the expression of ACTN2 and ACTN3 genes and proteins in the skeletal muscles. Am J Sports Sci Med 4:26–32. doi:10.12691/ajssm-4-2-1
Kim SJ, Ghaleh B, Kudej RK, Huang CH, Hintze TH, Vatner SF (1997) Delayed enhanced nitric oxide-mediated coronary vasodilation following brief ischemia and prolonged reperfusion in conscious dogs. Circ Res 81:53–59. doi:10.1161/01.RES.81.1.53
Kubon C, Mistry NB, Grundvold I, Halvorsen S, Kjeldsen SE, Westheim AS (2011) The role of beta-blockers in the treatment of chronic heart failure. Trends Pharmacol Sci 32:206–212. doi:10.1016/j.tips.2011.01.006
Lacerda AC, Marubayashi U, Balthazar CH, Leite LH, Coimbra CC (2006) Central nitric oxide inhibition modifies metabolic adjustments induced by exercise in rats. Neurosci Lett 410:152–156. doi:10.1016/j.neulet.2006.09.067
Lambernd S, Taube A, Schober A, Platzbecker B, Gorgens SW, Schlich R, Jeruschke K, Weiss J, Eckardt K, Eckel J (2012) Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia 55:1128–1139. doi:10.1007/s00125-012-2454-z
Langmead B, Trapnell C, Pop M, Salzberg SL (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. doi:10.1186/gb-2009-10-3-r25
Lawler JM, Kwak HB, Kim JH, Suk MH (2009) Exercise training inducibility of MnSOD protein expression and activity is retained while reducing prooxidant signaling in the heart of senescent rats. Am J Physiol Regul Integr Comp Physiol 296:R1496–R1502. doi:10.1152/ajpregu.90314.2008
Lee-Young RS, Ayala JE, Hunley CF, James FD, Bracy DP, Kang L, Wasserman DH (2010) Endothelial nitric oxide synthase is central to skeletal muscle metabolic regulation and enzymatic signaling during exercise in vivo. Am J Physiol Regul Integr Comp Physiol 298:R1399–R1408. doi:10.1152/ajpregu.00004.2010
Liu F, Chen Y, Feng X, Teng Z, Yuan Y, Bin J (2014) Effects of beta-blockers on heart failure with preserved ejection fraction: a meta-analysis. PLoS ONE 9:e90555. doi:10.1371/journal.pone.0090555
Martin WH 3rd, Coggan AR, Spina RJ, Saffitz JE (1989) Effects of fiber type and training on beta-adrenoceptor density in human skeletal muscle. Am J Physiol 257:E736–E742
Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K (2007) SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA 104:14855–14860. doi:10.1073/pnas.0704329104
McConell GK, Rattigan S, Lee-Young RS, Wadley GD, Merry TL (2012) Skeletal muscle nitric oxide signaling and exercise: a focus on glucose metabolism. Am J Physiol Endocrinol Metab 303:E301–E307. doi:10.1152/ajpendo.00667.2011
Michel-Reher MB, Michel MC (2015) Regulation of GAPDH expression by treatment with the beta-adrenoceptor agonist isoprenaline—is GADPH a suitable loading control in immunoblot experiments? Naunyn Schmiedebergs Arch Pharmacol 388:1119–1120. doi:10.1007/s00210-015-1166-6
Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO (2005) Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310:314–317. doi:10.1126/science.1117728
Ren WJ, Yang XD, Jiang XG, Li Z, Zhang ZX (2010) Chronic hypoxia and exercise training affect the NO content and NOS activity of rat skeletal muscle. Int Sportmed J 11:244–257
Rockl KS, Hirshman MF, Brandauer J, Fujii N, Witters LA, Goodyear LJ (2007) Skeletal muscle adaptation to exercise training: AMP-activated protein kinase mediates muscle fiber type shift. Diabetes 56:2062–2069
Tsukiyama Y, Ito T, Nagaoka K, Eguchi E, Ogino K (2017) Effects of exercise training on nitric oxide, blood pressure and antioxidant enzymes. J Clin Biochem Nutr 60:180–186. doi:10.3164/jcbn.16-108
Vatner DE, Yan L, Lai L, Yuan C, Mouchiroud L, Pachon RE, Zhang J, Dillinger JG, Houtkooper RH, Auwerx J, Vatner SF (2015) Type 5 adenylyl cyclase disruption leads to enhanced exercise performance. Aging Cell 14:1075–1084
Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM (2004) Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2:e294. doi:10.1371/journal.pbio.0020294
Williams RS, Caron MG, Daniel K (1984) Skeletal muscle beta-adrenergic receptors: variations due to fiber type and training. Am J Physiol 246:E160–E167
Wright DC, Han DH, Garcia-Roves PM, Geiger PC, Jones TE, Holloszy JO (2007) Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem 282:194–199. doi:10.1074/jbc.M606116200
Xia N, Strand S, Schlufter F, Siuda D, Reifenberg G, Kleinert H, Forstermann U, Li H (2013) Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide 32:29–35. doi:10.1016/j.niox.2013.04.001
Yan Z, Okutsu M, Akhtar YN, Lira VA (1985) Regulation of exercise-induced fiber type transformation, mitochondrial biogenesis, and angiogenesis in skeletal muscle. J Appl Physiol 110:264–274. doi:10.1152/japplphysiol.00993.2010
Young DR, Haskell WL, Jatulis DE, Fortmann SP (1993) Associations between changes in physical activity and risk factors for coronary heart disease in a community-based sample of men and women: the Stanford Five-City Project. Am J Epidemiol 138:205–216
Acknowledgements
We thank Dr. Gopal Babu for generating AC5 floxed mice. We thank Dr. Xiangzhen Sui for her work on the biochemical studies. We thank Dr. Yimin Tian for her work on the histological analyses.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
JJG, JZ, SCC, and MO declare that they have no conflict of interest. DEV and SFV have a company involved in developing AC5 inhibitors for cardioprotection. Exercise is not being pursued by this company and this entire study was supported by the NIH grants listed.
Disclosure
Drs. Dorothy and Stephen Vatner have a company involved in developing AC5 inhibitors for cardioprotection. Exercise is not being pursued by this company and this entire study was supported by the NIH grants listed.
Funding resources
This work was supported by National Institute of Health grants P01AG027211, R01HL102472, T32HL069752, P01HL069020, R01HL106511, R01HL093481, R01HL119464, R01HL124282, R01HL106511, and R01HL130848.
Rights and permissions
About this article
Cite this article
Guers, J.J., Zhang, J., Campbell, S.C. et al. Disruption of adenylyl cyclase type 5 mimics exercise training. Basic Res Cardiol 112, 59 (2017). https://doi.org/10.1007/s00395-017-0648-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-017-0648-8