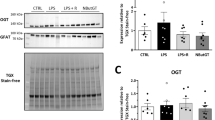
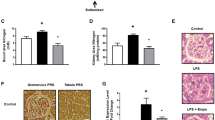
Abstract
Dipeptidyl peptidase (DPP)-4 inhibitors are used to treat hyperglycemia by increasing the incretin glucagon-like peptide-1 (GLP-1). Previous studies showed anti-inflammatory and antiatherosclerotic effects of DPP-4 inhibitors. Here, we compared the effects of linagliptin versus sitagliptin and liraglutide on survival and vascular function in animal models of endotoxic shock by prophylactic therapy and treatment after lipopolysaccharide (LPS) injection. Gliptins were administered either orally or subcutaneously: linagliptin (5 mg/kg/day), sitagliptin (50 mg/kg/day) or liraglutide (200 µg/kg/day). Endotoxic shock was induced by LPS injection (mice 17.5–20 mg/kg i.p., rats 10 mg/kg/day). Linagliptin and liraglutide treatment or DPP-4 knockout improved the survival of endotoxemic mice, while sitagliptin was ineffective. Linagliptin, liraglutide and sitagliptin ameliorated LPS-induced hypotension and vascular dysfunction in endotoxemic rats, suppressed inflammatory parameters such as whole blood nitrosyl-iron hemoglobin (leukocyte-inducible nitric oxide synthase activity) or aortic mRNA expression of markers of inflammation as well as whole blood and aortic reactive oxygen species formation. Hemostasis (tail bleeding time, activated partial thromboplastin time) was impaired in endotoxemic rats and recovered under cotreatment with linagliptin and liraglutide. Finally, the beneficial effects of linagliptin on vascular function and inflammatory parameters in endotoxemic mice were impaired in AMP-activated kinase (alpha1) knockout mice. The improved survival of endotoxemic animals and other data shown here may warrant further clinical evaluation of these drugs in patients with septic shock beyond the potential improvement of inflammatory complications in diabetic individuals with special emphasis on the role of AMP-activated kinase (alpha1) in the DPP-4/GLP-1 cascade.






Similar content being viewed by others
Abbreviations
- ACh:
-
Acetylcholine
- ADA:
-
Adenine deaminase
- AMPK:
-
AMP-activated protein kinase
- aPTT:
-
Activated partial thromboplastin time
- CCL-2:
-
See MCP-1
- DHE:
-
Dihydroethidine
- DPP-4:
-
Dipeptidyl peptidase-4
- ECL:
-
Enhanced chemiluminescence
- ecSOD:
-
Extracellular superoxide dismutase
- eNOS:
-
Endothelial •NO synthase (type 3)
- GLP-1:
-
Glucagon-like peptide-1
- ICAM-1:
-
Intercellular adhesion molecule-1
- IL-6:
-
Interleukin-6
- iNOS:
-
Inducible •NO synthase (type 2)
- L-012:
-
8-Amino-5-chloro-7-phenylpyrido[3,4-d]pyridazine-1,4-(2H,3H)dione sodium salt
- MCP-1:
-
Monocyte chemoattractant protein-1
- Nox:
-
Catalytic subunit of NADPH oxidase
- NIBP:
-
Non-invasive blood pressure
- qRT-PCR:
-
Quantitative reverse transcription polymerase chain reaction
- ROS:
-
Reactive oxygen species
- TNF-α:
-
Tumor necrosis factor-α
- VCAM-1:
-
Vascular adhesion molecule-1
References
Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A (2004) Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 89:2078–2084. doi:10.1210/jc.2003-031907
Ahren B, Pacini G, Foley JE, Schweizer A (2005) Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care 28:1936–1940. doi:10.2337/diacare.28.8.1936
Burgmaier M, Liberman A, Mollmann J, Kahles F, Reith S, Lebherz C, Marx N, Lehrke M (2013) Glucagon-like peptide-1 (GLP-1) and its split products GLP-1 (9–37) and GLP-1 (28–37) stabilize atherosclerotic lesions in apoe(−)/(−) mice. Atherosclerosis 231:427–435. doi:10.1016/j.atherosclerosis.2013.08.033
Burgmaier M, Vasic D, Ostertag R, Esser M, Walcher D, Ludwig A, Marx N, Vittone F (2011) Glucagon-like peptide-1(7-36)amide inhibits chemokine-induced monocyte migration and macrophage MMP-9 expression both in vitro and in ApoE−/− mice. Clin Res Cardiol 100:P694. doi:10.1007/s00392-011-1100-y
Busso N, Wagtmann N, Herling C, Chobaz-Peclat V, Bischof-Delaloye A, So A, Grouzmann E (2005) Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol 166:433–442. doi:10.1016/S0002-9440(10)62266-3
Chang SY, Kim DB, Ryu GR, Ko SH, Jeong IK, Ahn YB, Jo YH, Kim MJ (2013) Exendin-4 inhibits iNOS expression at the protein level in LPS-stimulated Raw264.7 macrophage by the activation of cAMP/PKA pathway. J Cell Biochem 114:844–853. doi:10.1002/jcb.24425
Daiber A, Oelze M, Coldewey M, Bachschmid M, Wenzel P, Sydow K, Wendt M, Kleschyov AL, Stalleicken D, Ullrich V, Mulsch A, Munzel T (2004) Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol Pharmacol 66:1372–1382. doi:10.1124/mol.104.002600
Dicembrini I, Pala L, Rotella CM (2011) From theory to clinical practice in the use of GLP-1 receptor agonists and DPP-4 inhibitors therapy. Exp Diabetes Res 2011:898913. doi:10.1155/2011/898913
Efron P, Moldawer LL (2003) Sepsis and the dendritic cell. Shock 20:386–401. doi:10.1097/01.SHK.0000092698.10326.6f
Fadini GP, Albiero M, Seeger F, Poncina N, Menegazzo L, Angelini A, Castellani C, Thiene G, Agostini C, Cappellari R, Boscaro E, Zeiher A, Dimmeler S, Avogaro A (2013) Stem cell compartmentalization in diabetes and high cardiovascular risk reveals the role of DPP-4 in diabetic stem cell mobilopathy. Basic Res Cardiol 108:313. doi:10.1007/s00395-012-0313-1
Feng M, Whitesall S, Zhang Y, Beibel M, D’Alecy L, DiPetrillo K (2008) Validation of volume–pressure recording tail-cuff blood pressure measurements. Am J Hypertens 21:1288–1291. doi:10.1038/ajh.2008.301
Gladwin MT, Ognibene FP, Pannell LK, Nichols JS, Pease-Fye ME, Shelhamer JH, Schechter AN (2000) Relative role of heme nitrosylation and beta-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. Proc Natl Acad Sci USA 97:9943–9948. doi:10.1073/pnas.180155397
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204:2449–2460. doi:10.1084/jem.20070657
Hausding M, Jurk K, Daub S, Kroller-Schon S, Stein J, Schwenk M, Oelze M, Mikhed Y, Kerahrodi JG, Kossmann S, Jansen T, Schulz E, Wenzel P, Reske-Kunz AB, Becker C, Munzel T, Grabbe S, Daiber A (2013) CD40L contributes to angiotensin II-induced pro-thrombotic state, vascular inflammation, oxidative stress and endothelial dysfunction. Basic Res Cardiol 108:386. doi:10.1007/s00395-013-0386-5
Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88:E14–E22. doi:10.1161/01.RES.88.2.e14
Iwaya H, Fujii N, Hagio M, Hara H, Ishizuka S (2013) Contribution of dipeptidyl peptidase IV to the severity of dextran sulfate sodium-induced colitis in the early phase. Biosci Biotechnol Biochem 77:1461–1466. doi:10.1271/bbb.130105
Jia L, Bonaventura C, Bonaventura J, Stamler JS (1996) S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380:221–226. doi:10.1038/380221a0
Kessler P, Bauersachs J, Busse R, Schini-Kerth VB (1997) Inhibition of inducible nitric oxide synthase restores endothelium-dependent relaxations in proinflammatory mediator-induced blood vessels. Arterioscler Thromb Vasc Biol 17:1746–1755. doi:10.1161/01.ATV.17.9.1746
Kirima K, Tsuchiya K, Sei H, Hasegawa T, Shikishima M, Motobayashi Y, Morita K, Yoshizumi M, Tamaki T (2003) Evaluation of systemic blood NO dynamics by EPR spectroscopy: HbNO as an endogenous index of NO. Am J Physiol Heart Circ Physiol 285:H589–H596. doi:10.1152/ajpheart.01010.2002
Krijnen PA, Hahn NE, Kholova I, Baylan U, Sipkens JA, van Alphen FP, Vonk AB, Simsek S, Meischl C, Schalkwijk CG, van Buul JD, van Hinsbergh VW, Niessen HW (2012) Loss of DPP4 activity is related to a prothrombogenic status of endothelial cells: implications for the coronary microvasculature of myocardial infarction patients. Basic Res Cardiol 107:233. doi:10.1007/s00395-011-0233-5
Kroller-Schon S, Knorr M, Hausding M, Oelze M, Schuff A, Schell R, Sudowe S, Scholz A, Daub S, Karbach S, Kossmann S, Gori T, Wenzel P, Schulz E, Grabbe S, Klein T, Munzel T, Daiber A (2012) Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc Res 96:140–149. doi:10.1093/cvr/cvs246
Kroller-Schon S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Munzel T, Daiber A (2014) Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal 20:247–266. doi:10.1089/ars.2012.4953
Ku HC, Chen WP, Su MJ (2010) GLP-1 signaling preserves cardiac function in endotoxemic Fischer 344 and DPP4-deficient rats. Naunyn Schmiedebergs Arch Pharmacol 382:463–474. doi:10.1007/s00210-010-0559-9
Laudes M, Oberhauser F, Schulte DM, Schilbach K, Freude S, Bilkovski R, Schulz O, Faust M, Krone W (2010) Dipeptidyl-peptidase 4 and attractin expression is increased in circulating blood monocytes of obese human subjects. Exp Clin Endocrinol Diabetes 118:473–477. doi:10.1055/s-0030-1249014
Levi M, Ten Cate H (1999) Disseminated intravascular coagulation. N Engl J Med 341:586–592. doi:10.1056/NEJM199908193410807
Liu L, Liu J, Tian XY, Wong WT, Lau CW, Xu A, Xu G, Ng CF, Yao X, Gao Y, Huang Y (2014) Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal 21:1571–1581. doi:10.1089/ars.2013.5519
Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N (2000) Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA 97:6874–6879. doi:10.1073/pnas.120069197
Marx N, Burgmaier M, Heinz P, Ostertag M, Hausauer A, Bach H, Durst R, Hombach V, Walcher D (2010) Glucagon-like peptide-1 (1–37) inhibits chemokine-induced migration of human CD4-positive lymphocytes. Cell Mol Life Sci 67:3549–3555. doi:10.1007/s00018-010-0396-5
Matheeussen V, Waumans Y, Martinet W, Van Goethem S, Van der Veken P, Scharpe S, Augustyns K, De Meyer GR, De Meester I (2013) Dipeptidyl peptidases in atherosclerosis: expression and role in macrophage differentiation, activation and apoptosis. Basic Res Cardiol 108:350. doi:10.1007/s00395-013-0350-4
Matsubara J, Sugiyama S, Sugamura K, Nakamura T, Fujiwara Y, Akiyama E, Kurokawa H, Nozaki T, Ohba K, Konishi M, Maeda H, Izumiya Y, Kaikita K, Sumida H, Jinnouchi H, Matsui K, Kim-Mitsuyama S, Takeya M, Ogawa H (2012) A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. J Am Coll Cardiol 59:265–276. doi:10.1016/j.jacc.2011.07.053
Moberly SP, Mather KJ, Berwick ZC, Owen MK, Goodwill AG, Casalini ED, Hutchins GD, Green MA, Ng Y, Considine RV, Perry KM, Chisholm RL, Tune JD (2013) Impaired cardiometabolic responses to glucagon-like peptide 1 in obesity and type 2 diabetes mellitus. Basic Res Cardiol 108:365. doi:10.1007/s00395-013-0365-x
Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T (2002) Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90:E58–E65. doi:10.1161/01.RES.0000012569.55432.02
Munzel T, Daiber A, Ullrich V, Mulsch A (2005) Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol 25:1551–1557. doi:10.1161/01.ATV.0000168896.64927.bb
Nabeno M, Akahoshi F, Kishida H, Miyaguchi I, Tanaka Y, Ishii S, Kadowaki T (2013) A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun 434:191–196. doi:10.1016/j.bbrc.2013.03.010
Oelze M, Daiber A, Brandes RP, Hortmann M, Wenzel P, Hink U, Schulz E, Mollnau H, von Sandersleben A, Kleschyov AL, Mulsch A, Li H, Forstermann U, Munzel T (2006) Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension 48:677–684. doi:10.1161/01.HYP.0000239207.82326.29
Oelze M, Knorr M, Schuhmacher S, Heeren T, Otto C, Schulz E, Reifenberg K, Wenzel P, Munzel T, Daiber A (2011) Vascular dysfunction in streptozotocin-induced experimental diabetes strictly depends on insulin deficiency. J Vasc Res 48:275–284. doi:10.1159/000320627
Patel GP, Balk RA (2007) Choice of vasopressor in septic shock: does it matter? Crit Care 11:174. doi:10.1186/cc6159
Schade J, Schmiedl A, Kehlen A, Veres TZ, Stephan M, Pabst R, von Horsten S (2009) Airway-specific recruitment of T cells is reduced in a CD26-deficient F344 rat substrain. Clin Exp Immunol 158:133–142. doi:10.1111/j.1365-2249.2009.03991.x
Schuhmacher S, Foretz M, Knorr M, Jansen T, Hortmann M, Wenzel P, Oelze M, Kleschyov AL, Daiber A, Keaney JF Jr, Wegener G, Lackner K, Munzel T, Viollet B, Schulz E (2011) alpha1AMP-activated protein kinase preserves endothelial function during chronic angiotensin II treatment by limiting Nox2 upregulation. Arterioscler Thromb Vasc Biol 31:560–566. doi:10.1161/ATVBAHA.110.219543
Schulz E, Dopheide J, Schuhmacher S, Thomas SR, Chen K, Daiber A, Wenzel P, Munzel T, Keaney JF Jr (2008) Suppression of the JNK pathway by induction of a metabolic stress response prevents vascular injury and dysfunction. Circulation 118:1347–1357. doi:10.1161/CIRCULATIONAHA.108.784298
Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T (2008) Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10:1115–1126. doi:10.1089/ars.2007.1989
Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R, Sun Q, Mihai G, Maiseyeu A, Rajagopalan S (2011) Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation 124:2338–2349. doi:10.1161/CIRCULATIONAHA.111.041418
Strassel C, Nonne C, Eckly A, David T, Leon C, Freund M, Cazenave JP, Gachet C, Lanza F (2007) Decreased thrombotic tendency in mouse models of the Bernard–Soulier syndrome. Arterioscler Thromb Vasc Biol 27:241–247. doi:10.1161/01.ATV.0000251992.47053.75
Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F, Mark M (2008) (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazoli n-2-ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther 325:175–182. doi:10.1124/jpet.107.135723
Vena GA, Vestita M, Cassano N (2010) Psoriasis and cardiovascular disease. Dermatol Ther 23:144–151. doi:10.1111/j.1529-8019.2010.01308.x
Wang Y, Parlevliet ET, Geerling JJ, van der Tuin SJ, Zhang H, Bieghs V, Jawad AH, Shiri-Sverdlov R, Bot I, de Jager SC, Havekes LM, Romijn JA, Willems van Dijk K, Rensen PC (2014) Exendin-4 decreases liver inflammation and atherosclerosis development simultaneously by reducing macrophage infiltration. Br J Pharmacol 171:723–734. doi:10.1111/bph.12490
Wenzel P, Hink U, Oelze M, Seeling A, Isse T, Bruns K, Steinhoff L, Brandt M, Kleschyov AL, Schulz E, Lange K, Weiner H, Lehmann J, Lackner KJ, Kawamoto T, Munzel T, Daiber A (2007) Number of nitrate groups determines reactivity and potency of organic nitrates: a proof of concept study in ALDH-2−/− mice. Brit J Pharmacol 150:526–533. doi:10.1038/sj.bjp.0707116
Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Munzel T (2011) Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124:1370–1381. doi:10.1161/CIRCULATIONAHA.111.034470
Wenzel P, Schulz E, Oelze M, Muller J, Schuhmacher S, Alhamdani MS, Debrezion J, Hortmann M, Reifenberg K, Fleming I, Munzel T, Daiber A (2008) AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic Biol Med 45:619–626. doi:10.1016/j.freeradbiomed.2008.05.009
Xing J, Wang Q, Coughlan K, Viollet B, Moriasi C, Zou MH (2013) Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. Am J Pathol 182:1021–1030. doi:10.1016/j.ajpath.2012.11.022
Yan W, Zhang H, Liu P, Wang H, Liu J, Gao C, Liu Y, Lian K, Yang L, Sun L, Guo Y, Zhang L, Dong L, Lau WB, Gao E, Gao F, Xiong L, Wang H, Qu Y, Tao L (2013) Impaired mitochondrial biogenesis due to dysfunctional adiponectin-AMPK-PGC-1alpha signaling contributing to increased vulnerability in diabetic heart. Basic Res Cardiol 108:329. doi:10.1007/s00395-013-0329-1
Zhong J, Rao X, Deiuliis J, Braunstein Z, Narula V, Hazey J, Mikami D, Needleman B, Satoskar AR, Rajagopalan S (2013) A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes 62:149–157. doi:10.2337/db12-0230
Zhong J, Rao X, Rajagopalan S (2013) An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis 226:305–314. doi:10.1016/j.atherosclerosis.2012.09.012
Acknowledgments
We are indebted to Angelica Karpi, Jörg Schreiner, Jessica Rudolph, Bettina Mros and Evelyn Montermann for expert technical assistance. This paper contains results that are part of the doctoral thesis of Michael Mader. The present work was supported by vascular biology research grants from Boehringer Ingelheim Pharma GmbH & Co. KG (A.D. and T.M.). Sebastian Steven holds a Virchow-Fellowship from the Center of Thrombosis and Hemostasis (Mainz, Germany) funded by the Federal Ministry of Education and Research (BMBF 01EO1003). Yuliya Miked holds a stipend from the International PhD Programme on the “Dynamics of Gene Regulation, Epigenetics and DNA Damage Response” from the Institute of Molecular Biology gGmbH, (Mainz, Germany) funded by the Boehringer Ingelheim Foundation.
Conflict of interest
A.D. and T.M. received research grant support from Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany. T.K. is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Ingelheim, Germany. All other authors have no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Steven and M. Hausding contributed equally to this study and should therefore both be considered as first author.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Steven, S., Hausding, M., Kröller-Schön, S. et al. Gliptin and GLP‐1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide‐induced endotoxemia. Basic Res Cardiol 110, 6 (2015). https://doi.org/10.1007/s00395-015-0465-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-015-0465-x