Abstract
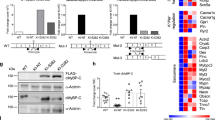
Hypertrophic cardiomyopathy (HCM) is a myocardial disease associated with mutations in sarcomeric genes. Three mutations were found in ANKRD1, encoding ankyrin repeat domain 1 (ANKRD1), a transcriptional co-factor located in the sarcomere. In the present study, we investigated whether expression of HCM-associated ANKRD1 mutations affects contraction parameters after gene transfer in engineered heart tissues (EHTs). EHTs were generated from neonatal rat heart cells and were transduced with adeno-associated virus encoding GFP or myc-tagged wild-type (WT) or mutant (P52A, T123M, or I280V) ANKRD1. Contraction parameters were analyzed from day 8 to day 16 of culture, and evaluated in the absence or presence of the proteasome inhibitor epoxomicin for 24 h. Under standard conditions, only WT- and T123M-ANKRD1 were correctly incorporated in the sarcomere. T123M-ANKRD1-transduced EHTs exhibited higher force and velocities of contraction and relaxation than WT- P52A- and I280V-ANKRD1 were highly unstable, not incorporated into the sarcomere, and did not induce contractile alterations. After epoxomicin treatment, P52A and I280V were both stabilized and incorporated into the sarcomere. I280V-transduced EHTs showed prolonged relaxation. These data suggest different impacts of ANKRD1 mutations on cardiomyocyte function: gain-of-function for T123M mutation under all conditions and dominant-negative effect for the I280V mutation which may come into play only when the proteasome is impaired.








Similar content being viewed by others
References
Ahmad F, Seidman JG, Seidman CE (2005) The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet 6:185–216. doi:10.1146/annurev.genom.6.080604.162132
Aihara Y, Kurabayashi M, Saito Y, Ohyama Y, Tanaka T, Takeda S, Tomaru K, Sekiguchi K, Arai M, Nakamura T, Nagai R (2000) Cardiac ankyrin repeat protein is a novel marker of cardiac hypertrophy: role of M-CAT element within the promoter. Hypertension 36:48–53
Arimura T, Bos JM, Sato A, Kubo T, Okamoto H, Nishi H, Harada H, Koga Y, Moulik M, Doi YL, Towbin JA, Ackerman MJ, Kimura A (2009) Cardiac ankyrin repeat protein gene (ANKRD1) mutations in hypertrophic cardiomyopathy. J Am Coll Cardiol 54:334–342. doi:10.1016/j.jacc.2008.12.082
Badi I, Cinquetti R, Frascoli M, Parolini C, Chiesa G, Taramelli R, Acquati F (2009) Intracellular ANKRD1 protein levels are regulated by 26S proteasome-mediated degradation. FEBS Lett 583:2486–2492. doi:10.1016/j.febslet.2009.07.001
Bang ML, Mudry RE, McElhinny AS, Trombitas K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S (2001) Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol 153:413–427
Barash IA, Bang ML, Mathew L, Greaser ML, Chen J, Lieber RL (2007) Structural and regulatory roles of muscle ankyrin repeat protein family in skeletal muscle. Am J Physiol Cell Physiol 293:C218–C227. doi:10.1152/ajpcell.00055.2007
Barefield D, Sadayappan S (2010) Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J Mol Cell Cardiol 48:866–875. doi:10.1016/j.yjmcc.2009.11.014
Baudet S (2003) Another activity for the cardiac biologist: CARP fishing. Cardiovasc Res 59:529–531 ([pii] S0008636303005030)
Cazorla O, Szilagyi S, Vignier N, Salazar G, Kramer E, Vassort G, Carrier L, Lacampagne A (2006) Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovasc Res 69:370–380. doi:10.1016/j.cardiores.2005.11.009
Chen B, Zhong L, Roush SF, Pentassuglia L, Peng X, Samaras S, Davidson JM, Sawyer DB, Lim CC (2012) Disruption of a GATA4/Ankrd1 signaling axis in cardiomyocytes leads to sarcomere disarray: implications for anthracycline cardiomyopathy. PLoS ONE 7:e35743. doi:10.1371/journal.pone.0035743
Cinquetti R, Badi I, Campione M, Bortoletto E, Chiesa G, Parolini C, Camesasca C, Russo A, Taramelli R, Acquati F (2008) Transcriptional deregulation and a missense mutation define ANKRD1 as a candidate gene for total anomalous pulmonary venous return. Hum Mutat 29:468–474. doi:10.1002/humu.20711
Fraysse B, Weinberger F, Bardswell SC, Cuello F, Vignier N, Geertz B, Starbatty J, Kramer E, Coirault C, Eschenhagen T, Kentish JC, Avkiran M, Carrier L (2012) Increased myofilament Ca(2+) sensitivity and diastolic dysfunction as early consequences of Mybpc3 mutation in heterozygous knock-in mice. J Mol Cell Cardiol 52:1299–1307. doi:10.1016/j.yjmcc.2012.03.009
Friedrich FW, Carrier L (2012) Genetics of hypertrophic and dilated cardiomyopathy. Curr Pharm Biotechnol 13:2467–2476 ([pii] CPB-EPUB-20120120-006)
Friedrich FW, Wilding BR, Reischmann S, Crocini C, Lang P, Charron P, Muller OJ, McGrath MJ, Vollert I, Hansen A, Linke WA, Hengstenberg C, Bonne G, Morner S, Wichter T, Madeira H, Arbustini E, Eschenhagen T, Mitchell CA, Isnard R, Carrier L (2012) Evidence for FHL1 as a novel disease gene for isolated hypertrophic cardiomyopathy. Hum Mol Genet 21:3237–3254. doi:10.1093/hmg/dds157
Hansen A, Eder A, Bonstrup M, Flato M, Mewe M, Schaaf S, Aksehirlioglu B, Schwoerer AP, Uebeler J, Eschenhagen T (2010) Development of a drug screening platform based on engineered heart tissue. Circ Res 107:35–44. doi:10.1161/CIRCRESAHA.109.211458
Hirt MN, Sorensen NA, Bartholdt LM, Boeddinghaus J, Schaaf S, Eder A, Vollert I, Stohr A, Schulze T, Witten A, Stoll M, Hansen A, Eschenhagen T (2012) Increased afterload induces pathological cardiac hypertrophy: a new in vitro model. Basic Res Cardiol 107:307. doi:10.1007/s00395-012-0307-z
Ihara Y, Suzuki YJ, Kitta K, Jones LR, Ikeda T (2002) Modulation of gene expression in transgenic mouse hearts overexpressing calsequestrin. Cell Calcium 32:21–29 ([pii] S0143416002000969)
Jeyaseelan R, Poizat C, Baker RK, Abdishoo S, Isterabadi LB, Lyons GE, Kedes L (1997) A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem 272:22800–22808
Kimura A (2010) Molecular basis of hereditary cardiomyopathy: abnormalities in calcium sensitivity, stretch response, stress response and beyond. J Hum Genet 55:81–90. doi:10.1038/jhg.2009.138
Kuo H, Chen J, Ruiz-Lozano P, Zou Y, Nemer M, Chien KR (1999) Control of segmental expression of the cardiac-restricted ankyrin repeat protein gene by distinct regulatory pathways in murine cardiogenesis. Development 126:4223–4234
Laure L, Daniele N, Suel L, Marchand S, Aubert S, Bourg N, Roudaut C, Duguez S, Bartoli M, Richard I (2010) A new pathway encompassing calpain 3 and its newly identified substrate cardiac ankyrin repeat protein is involved in the regulation of the nuclear factor-kappaB pathway in skeletal muscle. FEBS J 277:4322–4337. doi:10.1111/j.1742-4658.2010.07820.x
Linke WA (2008) Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res 77:637–648. doi:10.1016/j.cardiores.2007.03.029
Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113:1807–1816. doi:10.1161/CIRCULATIONAHA.106.174287
Mikhailov AT, Torrado M (2008) The enigmatic role of the ankyrin repeat domain 1 gene in heart development and disease. Int J Dev Biol 52:811–821. doi:10.1387/ijdb.082655am
Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC, Labeit S (2003) The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol 333:951–964 ([pii] S0022283603011380)
Morimoto S, Yanaga F, Minakami R, Ohtsuki I (1998) Ca2+-sensitizing effects of the mutations at Ile-79 and Arg-92 of troponin T in hypertrophic cardiomyopathy. Am J Physiol 275:C200–C207
Moulik M, Vatta M, Witt SH, Arola AM, Murphy RT, McKenna WJ, Boriek AM, Oka K, Labeit S, Bowles NE, Arimura T, Kimura A, Towbin JA (2009) ANKRD1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J Am Coll Cardiol 54:325–333. doi:10.1016/j.jacc.2009.02.076
Muller OJ, Schinkel S, Kleinschmidt JA, Katus HA, Bekeredjian R (2008) Augmentation of AAV-mediated cardiac gene transfer after systemic administration in adult rats. Gene Ther 15:1558–1565. doi:10.1038/gt.2008.111
Neulen A, Stehle R, Pfitzer G (2009) The cardiac troponin C mutation Leu29Gln found in a patient with hypertrophic cardiomyopathy does not alter contractile parameters in skinned murine myocardium. Basic Res Cardiol 104:751–760. doi:10.1007/s00395-009-0038-y
Pohlmann L, Kroger I, Vignier N, Schlossarek S, Kramer E, Coirault C, Sultan KR, El-Armouche A, Winegrad S, Eschenhagen T, Carrier L (2007) Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ Res 101:928–938. doi:10.1161/CIRCRESAHA.107.158774
Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM (2010) Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation 121:997–1004. doi:10.1161/CIRCULATIONAHA.109.904557
Purevjav E, Arimura T, Augustin S, Huby AC, Takagi K, Nunoda S, Kearney DL, Taylor MD, Terasaki F, Bos JM, Ommen SR, Shibata H, Takahashi M, Itoh-Satoh M, McKenna WJ, Murphy RT, Labeit S, Yamanaka Y, Machida N, Park JE, Alexander PM, Weintraub RG, Kitaura Y, Ackerman MJ, Kimura A, Towbin JA (2012) Molecular basis for clinical heterogeneity in inherited cardiomyopathies due to myopalladin mutations. Hum Mol Genet 21:2039–2053. doi:10.1093/hmg/dds022
Rechsteiner M, Rogers SW (1996) PEST sequences and regulation by proteolysis. Trends Biochem Sci 21:267–271 ([pii] S0968-0004(96)10031-1)
Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P (1996) Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 93:841–842
Robinson P, Griffiths PJ, Watkins H, Redwood CS (2007) Dilated and hypertrophic cardiomyopathy mutations in troponin and alpha-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ Res 101:1266–1273
Samaras SE, Chen B, Koch SR, Sawyer DB, Lim CC, Davidson JM (2012) 26S proteasome regulation of Ankrd1/CARP in adult rat ventricular myocytes and human microvascular endothelial cells. Biochem Biophys Res Commun 425:830–835. doi:10.1016/j.bbrc.2012.07.162
Schlossarek S, Englmann DR, Sultan KR, Sauer M, Eschenhagen T, Carrier L (2012) Defective proteolytic systems in Mybpc3-targeted mice with cardiac hypertrophy. Basic Res Cardiol 107:235. doi:10.1007/s00395-011-0235-3
Schlossarek S, Mearini G, Carrier L (2011) Cardiac myosin-binding protein C in hypertrophic cardiomyopathy: mechanisms and therapeutic opportunities. J Mol Cell Cardiol 50:613–620. doi:10.1016/j.yjmcc.2011.01.014
Schlossarek S, Schuermann F, Geertz B, Mearini G, Eschenhagen T, Carrier L (2012) Adrenergic stress reveals septal hypertrophy and proteasome impairment in heterozygous Mybpc3-targeted knock-in mice. J Muscle Res Cell Motil 33:5–15. doi:10.1007/s10974-011-9273-6
Tripathi S, Schultz I, Becker E, Montag J, Borchert B, Francino A, Navarro-Lopez F, Perrot A, Ozcelik C, Osterziel KJ, McKenna WJ, Brenner B, Kraft T (2011) Unequal allelic expression of wild-type and mutated beta-myosin in familial hypertrophic cardiomyopathy. Basic Res Cardiol 106:1041–1055. doi:10.1007/s00395-011-0205-9
Tsukamoto O, Minamino T, Kitakaze M (2010) Functional alterations of cardiac proteasomes under physiological and pathological conditions. Cardiovasc Res 85:339–346. doi:10.1093/cvr/cvp282
van Dijk SJ, Dooijes D, dos Remedios C, Michels M, Lamers JM, Winegrad S, Schlossarek S, Carrier L, ten Cate FJ, Stienen GJ, van der Velden J (2009) Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation 119:1473–1483. doi:10.1161/CIRCULATIONAHA.108.838672
van Dijk SJ, Paalberends ER, Najafi A, Michels M, Sadayappan S, Carrier L, Boontje NM, Kuster DW, van Slegtenhorst M, Dooijes D, Dos Remedios C, Ten Cate FJ, Stienen GJ, van der Velden J (2012) Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ Heart Fail 5:36–46. doi:10.1161/CIRCHEARTFAILURE.111.963702
Vandenburgh H, Shansky J, Benesch-Lee F, Barbata V, Reid J, Thorrez L, Valentini R, Crawford G (2008) Drug-screening platform based on the contractility of tissue-engineered muscle. Muscle Nerve 37:438–447. doi:10.1002/mus.20931
Vignier N, Schlossarek S, Fraysse B, Mearini G, Kramer E, Pointu H, Mougenot N, Guiard J, Reimer R, Hohenberg H, Schwartz K, Vernet M, Eschenhagen T, Carrier L (2009) Nonsense-mediated mRNA decay and ubiquitin-proteasome system regulate cardiac myosin-binding protein C mutant levels in cardiomyopathic mice. Circ Res 105:239–248. doi:10.1161/CIRCRESAHA.109.201251
Witt CC, Witt SH, Lerche S, Labeit D, Back W, Labeit S (2008) Cooperative control of striated muscle mass and metabolism by MuRF1 and MuRF2. EMBO J 27:350–360. doi:10.1038/sj.emboj.7601952
Witt SH, Labeit D, Granzier H, Labeit S, Witt CC (2005) Dimerization of the cardiac ankyrin protein CARP: implications for MARP titin-based signaling. J Muscle Res Cell Motil 26:401–408. doi:10.1007/s10974-005-9022-9
Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T (2000) Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng 68:106–114. doi:10.1002/(SICI)1097-0290(20000405)68:1<106::AID-BIT13>3.0.CO;2-3
Zolk O, Frohme M, Maurer A, Kluxen FW, Hentsch B, Zubakov D, Hoheisel JD, Zucker IH, Pepe S, Eschenhagen T (2002) Cardiac ankyrin repeat protein, a negative regulator of cardiac gene expression, is augmented in human heart failure. Biochem Biophys Res Commun 293:1377–1382. doi:10.1016/S0006-291X(02)00387-X
Zou Y, Evans S, Chen J, Kuo HC, Harvey RP, Chien KR (1997) CARP, a cardiac ankyrin repeat protein, is downstream in the Nk2–5 homeobox gene pathway. Development 124:793–804
Acknowledgments
We would like to thank Sebastian Schaaf (Hamburg) for technical help in video-optical recordings of EHTs, Oliver Müller (Heidelberg, Germany) for providing the dsAAV-CMVenh-MLC260-EGFP plasmid, and Evelyn Bendrat (HEXT, Hamburg) for production of AAVs. We would also like to thank S. Sadayappan (Chicago, OH, USA) and S. Labeit (Mannheim, Germany) for the anti-cMyBP-C antibody and the anti-ANKRD1 antibody, respectively. This work was supported in part by Grant-in-Aids for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, a research grant on measures of intractable diseases from the Ministry of Health, Labour and Welfare, Japan, a research grant from the Association Française contre les Myopathie, follow-up grants provided from the Tokyo Medical and Dental University, Joint Usage/Research Program of Medical Research Institute Tokyo Medical and Dental University, as well as by the seventh Framework Program of the European Union (Health-F2-2009-241577; Big-Heart project).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
C. Crocini and T. Arimura contributed equally to this work.
Rights and permissions
About this article
Cite this article
Crocini, C., Arimura, T., Reischmann, S. et al. Impact of ANKRD1 mutations associated with hypertrophic cardiomyopathy on contraction parameters of engineered heart tissue. Basic Res Cardiol 108, 349 (2013). https://doi.org/10.1007/s00395-013-0349-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0349-x