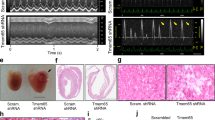
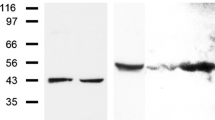
Abstract
The cardiac intercalated disc harbors mechanical and electrical junctions as well as ion channel complexes mediating propagation of electrical impulses. Cardiac connexin43 (Cx43) co-localizes and interacts with several of the proteins located at intercalated discs in the ventricular myocardium. We have generated conditional Cx43D378stop mice lacking the last five C-terminal amino acid residues, representing a binding motif for zonula occludens protein-1 (ZO-1), and investigated the functional consequences of this mutation on cardiac physiology and morphology. Newborn and adult homozygous Cx43D378stop mice displayed markedly impaired and heterogeneous cardiac electrical activation properties and died from severe ventricular arrhythmias. Cx43 and ZO-1 were co-localized at intercalated discs in Cx43D378stop hearts, and the Cx43D378stop gap junction channels showed normal coupling properties. Patch clamp analyses of isolated adult Cx43D378stop cardiomyocytes revealed a significant decrease in sodium and potassium current densities. Furthermore, we also observed a significant loss of Nav1.5 protein from intercalated discs in Cx43D378stop hearts. The phenotypic lethality of the Cx43D378stop mutation was very similar to the one previously reported for adult Cx43 deficient (Cx43KO) mice. Yet, in contrast to Cx43KO mice, the Cx43 gap junction channel was still functional in the Cx43D378stop mutant. We conclude that the lethality of Cx43D378stop mice is independent of the loss of gap junctional intercellular communication, but most likely results from impaired cardiac sodium and potassium currents. The Cx43D378stop mice reveal for the first time that Cx43 dependent arrhythmias can develop by mechanisms other than impairment of gap junction channel function.







Similar content being viewed by others
References
Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD (1997) Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100:169–179. doi:10.1172/JCI119509
Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE (2009) A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 360:1075–1084. doi:10.1056/NEJMoa0808138
Boengler K, Dodoni G, Rodriguez-Sinovas A, Cabestrero A, Ruiz-Meana M, Gres P, Konietzka I, Lopez-Iglesias C, Garcia-Dorado D, Di Lisa F, Heusch G, Schulz R (2005) Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc Res 67:234–244. doi:10.1016/j.cardiores.2005.04.014
Bruce AF, Rothery S, Dupont E, Severs NJ (2008) Gap junction remodelling in human heart failure is associated with increased interaction of connexin 43 with ZO-1. Cardiovasc Res 77:757–765. doi:10.1093/cvr/cvm083
Bukauskas FF, Verselis VK (2004) Gap junction channel gating. Biochim Biophys Acta 1662:42–60. doi:10.1016/j.bbamem.2004.01.008
Casini S, Tan HL, Demirayak I, Remme CA, Amin AS, Scicluna BP, Chatyan H, Ruijter JM, Bezzina CR, van Ginneken AC, Veldkamp MW (2010) Tubulin polymerization modifies cardiac sodium channel expression and gating. Cardiovasc Res 85:691–700. doi:10.1093/cvr/cvp352
Cheng L, Yung A, Covarrubias M, Radice GL (2011) Cortactin is required for N-cadherin regulation of Kv 1.5 channel function. J Biol Chem 286:20478–20489. doi:10.1074/jbc.M111.218560
Chkourko HS, Guerrero-Serna G, Lin X, Darwish N, Pohlmann JR, Cook KE, Martens JR, Rothenberg E, Musa H, Delmar M (2012) Remodeling of mechanical junctions and of microtubule-associated proteins accompany cardiac connexin43 lateralization. Heart Rhythm 9(1133–1140):e1136. doi:10.1016/j.hrthm.2012.03.003
Danik SB, Rosner G, Lader J, Gutstein DE, Fishman GI, Morley GE (2008) Electrical remodeling contributes to complex tachyarrhythmias in connexin43-deficient mouse hearts. FASEB J 22:1204–1212. doi:10.1096/fj.07-8974com
Delmar M (2012) Connexin43 regulates sodium current; ankyrin-G modulates gap junctions: the intercalated disc exchanger. Cardiovasc Res 93:220–222. doi:10.1093/cvr/cvr343
Delmar M (2012) Desmosome-ion channel interactions and their possible role in arrhythmogenic cardiomyopathy. Pediatr Cardiol 33:975–979. doi:10.1007/s00246-012-0257-0
Delmar M, Liang FX (2012) Connexin43 and the regulation of intercalated disc function. Heart Rhythm 9:835–838. doi:10.1016/j.hrthm.2011.10.028
Desplantez T, McCain ML, Beauchamp P, Rigoli G, Rothen-Rutishauser B, Parker KK, Kleber AG (2012) Connexin43 ablation in foetal atrial myocytes decreases electrical coupling, partner connexins, and sodium current. Cardiovasc Res 94:58–65. doi:10.1093/cvr/cvs025
Eckardt D, Kirchhoff S, Kim JS, Degen J, Theis M, Ott T, Wiesmann F, Doevendans PA, Lamers WH, de Bakker JM, van Rijen HV, Schneider MD, Willecke K (2006) Cardiomyocyte-restricted deletion of connexin43 during mouse development. J Mol Cell Cardiol 41:963–971. doi:10.1016/j.yjmcc.2006.07.017
Eckardt D, Theis M, Degen J, Ott T, van Rijen HV, Kirchhoff S, Kim JS, de Bakker JM, Willecke K (2004) Functional role of connexin43 gap junction channels in adult mouse heart assessed by inducible gene deletion. J Mol Cell Cardiol 36:101–110
Fontes MS, van Veen TA, de Bakker JM, van Rijen HV (2012) Functional consequences of abnormal Cx43 expression in the heart. Biochim Biophys Acta 1818:2020–2029. doi:10.1016/j.bbamem.2011.07.039
Francis R, Xu X, Park H, Wei CJ, Chang S, Chatterjee B, Lo C (2011) Connexin43 modulates cell polarity and directional cell migration by regulating microtubule dynamics. PLoS ONE 6:e26379. doi:10.1371/journal.pone.0026379
Franke WW, Schumacher H, Borrmann CM, Grund C, Winter-Simanowski S, Schlechter T, Pieperhoff S, Hofmann I (2007) The area composita of adhering junctions connecting heart muscle cells of vertebrates-III: assembly and disintegration of intercalated disks in rat cardiomyocytes growing in culture. Eur J Cell Biol 86:127–142. doi:10.1016/j.ejcb.2006.11.003
Giepmans BN, Moolenaar WH (1998) The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol 8:931–934
Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, Garcia-Dorado D, Di Lisa F, Schulz R, Heusch G (2005) Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res 97:583–586. doi:10.1161/01.RES.0000181171.65293.65
Herr C, Smyth N, Ullrich S, Yun F, Sasse P, Hescheler J, Fleischmann B, Lasek K, Brixius K, Schwinger RH, Fassler R, Schroder R, Noegel AA (2001) Loss of annexin A7 leads to alterations in frequency-induced shortening of isolated murine cardiomyocytes. Mol Cell Biol 21:4119–4128. doi:10.1128/MCB.21.13.4119-4128.2001
Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL (2007) Characterization of the pH-dependent interaction between the gap junction protein connexin43 carboxyl terminus and cytoplasmic loop domains. J Biol Chem 282:5801–5813. doi:10.1074/jbc.M605233200
Hund TJ, Koval OM, Li J, Wright PJ, Qian L, Snyder JS, Gudmundsson H, Kline CF, Davidson NP, Cardona N, Rasband MN, Anderson ME, Mohler PJ (2010) A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest 120:3508–3519. doi:10.1172/JCI43621
Hunter AW, Barker RJ, Zhu C, Gourdie RG (2005) Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 16:5686–5698. doi:10.1091/mbc.E05-08-0737
Itoh M, Nagafuchi A, Moroi S, Tsukita S (1997) Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol 138:181–192
Jansen JA, Noorman M, Musa H, Stein M, de Jong S, van der Nagel R, Hund TJ, Mohler PJ, Vos MA, van Veen TA, de Bakker JM, Delmar M, van Rijen HV (2012) Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm 9:600–607. doi:10.1016/j.hrthm.2011.11.025
Kanaporis G, Brink PR, Valiunas V (2011) Gap junction permeability: selectivity for anionic and cationic probes. Am J Physiol Cell Physiol 300:C600–C609. doi:10.1152/ajpcell.00316.2010
Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, Squarcioni CP, McKenna WJ, Thiene G, Basso C, Brousse N, Fontaine G, Saffitz JE (2004) Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease). Heart Rhythm 1:3–11. doi:10.1016/j.hrthm.2004.01.001
Kieken F, Mutsaers N, Dolmatova E, Virgil K, Wit AL, Kellezi A, Hirst-Jensen BJ, Duffy HS, Sorgen PL (2009) Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res 104:1103–1112. doi:10.1161/CIRCRESAHA.108.190454
Koval OM, Snyder JS, Wolf RM, Pavlovicz RE, Glynn P, Curran J, Leymaster ND, Dun W, Wright PJ, Cardona N, Qian L, Mitchell CC, Boyden PA, Binkley PF, Li C, Anderson ME, Mohler PJ, Hund TJ (2012) Ca2+/calmodulin-dependent protein kinase II-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation 126:2084–2094. doi:10.1161/CIRCULATIONAHA.112.105320
Kucera JP, Rohr S, Rudy Y (2002) Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res 91:1176–1182
Lammers WJ, Schalij MJ, Kirchhof CJ, Allessie MA (1990) Quantification of spatial inhomogeneity in conduction and initiation of reentrant atrial arrhythmias. Am J Physiol 259:H1254–H1263
Li J, Goossens S, van Hengel J, Gao E, Cheng L, Tyberghein K, Shang X, De Rycke R, van Roy F, Radice GL (2012) Loss of alphaT-catenin alters the hybrid adhering junctions in the heart and leads to dilated cardiomyopathy and ventricular arrhythmia following acute ischemia. J Cell Sci 125:1058–1067. doi:10.1242/jcs.098640
Lin X, Gemel J, Beyer EC, Veenstra RD (2005) Dynamic model for ventricular junctional conductance during the cardiac action potential. Am J Physiol Heart Circ Physiol 288:H1113–H1123. doi:10.1152/ajpheart.00882.2004
Lin X, Liu N, Lu J, Zhang J, Anumonwo JM, Isom LL, Fishman GI, Delmar M (2011) Subcellular heterogeneity of sodium current properties in adult cardiac ventricular myocytes. Heart Rhythm 8:1923–1930. doi:10.1016/j.hrthm.2011.07.016
Maass K, Chase SE, Lin X, Delmar M (2009) Cx43 CT domain influences infarct size and susceptibility to ventricular tachyarrhythmias in acute myocardial infarction. Cardiovasc Res 84:361–367. doi:10.1093/cvr/cvp250
Maass K, Ghanem A, Kim JS, Saathoff M, Urschel S, Kirfel G, Grummer R, Kretz M, Lewalter T, Tiemann K, Winterhager E, Herzog V, Willecke K (2004) Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell 15:4597–4608. doi:10.1091/mbc.E04-04-0324
Maass K, Shibayama J, Chase SE, Willecke K, Delmar M (2007) C-terminal truncation of connexin43 changes number, size, and localization of cardiac gap junction plaques. Circ Res 101:1283–1291. doi:10.1161/CIRCRESAHA.107.162818
Malhotra JD, Thyagarajan V, Chen C, Isom LL (2004) Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J Biol Chem 279:40748–40754. doi:10.1074/jbc.M407243200
Murray KT, Anno T, Bennett PB, Hondeghem LM (1990) Voltage clamp of the cardiac sodium current at 37 °C in physiologic solutions. Biophys J 57:607–613. doi:10.1016/S0006-3495(90)82576-5
O’Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG (2011) A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res 108:704–715. doi:10.1161/CIRCRESAHA.110.235747
Peters NS, Green CR, Poole-Wilson PA, Severs NJ (1993) Reduced content of connexin43 gap junctions in ventricular myocardium from hypertrophied and ischemic human hearts. Circulation 88:864–875
Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H (2011) SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res 108:294–304. doi:10.1161/CIRCRESAHA.110.228312
Rackauskas M, Kreuzberg MM, Pranevicius M, Willecke K, Verselis VK, Bukauskas FF (2007) Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys J 92:1952–1965. doi:10.1529/biophysj.106.099358
Rackauskas M, Verselis VK, Bukauskas FF (2007) Permeability of homotypic and heterotypic gap junction channels formed of cardiac connexins mCx30.2, Cx40, Cx43, and Cx45. Am J Physiol Heart Circ Physiol 293:H1729–H1736. doi:10.1152/ajpheart.00234.2007
Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J (1995) Cardiac malformation in neonatal mice lacking connexin43. Science 267:1831–1834
Rhett JM, Jourdan J, Gourdie RG (2011) Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell 22:1516–1528. doi:10.1091/mbc.E10-06-0548
Rhett JM, Ongstad EL, Jourdan J, Gourdie RG (2012) Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol 245:411–422. doi:10.1007/s00232-012-9465-z
Sato PY, Coombs W, Lin X, Nekrasova O, Green KJ, Isom LL, Taffet SM, Delmar M (2011) Interactions between ankyrin-G, plakophilin-2, and connexin43 at the cardiac intercalated disc. Circ Res 109:193–201. doi:10.1161/CIRCRESAHA.111.247023
Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patino GA, Taffet SM, Isom LL, Delmar M (2009) Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res 105:523–526. doi:10.1161/CIRCRESAHA.109.201418
Schrickel JW, Brixius K, Herr C, Clemen CS, Sasse P, Reetz K, Grohe C, Meyer R, Tiemann K, Schroder R, Bloch W, Nickenig G, Fleischmann BK, Noegel AA, Schwinger RH, Lewalter T (2007) Enhanced heterogeneity of myocardial conduction and severe cardiac electrical instability in annexin A7-deficient mice. Cardiovasc Res 76:257–268. doi:10.1016/j.cardiores.2007.07.001
Severs NJ (2007) The carboxy terminal domain of connexin43: from molecular regulation of the gap junction channel to supramolecular organization of the intercalated disk. Circ Res 101:1213–1215. doi:10.1161/CIRCRESAHA.107.165662
Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY (2007) Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128:547–560. doi:10.1016/j.cell.2006.12.037
Sorgen PL, Duffy HS, Sahoo P, Coombs W, Delmar M, Spray DC (2004) Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J Biol Chem 279:54695–54701. doi:10.1074/jbc.M409552200
Takefuji M, Wirth A, Lukasova M, Takefuji S, Boettger T, Braun T, Althoff T, Offermanns S, Wettschureck N (2012) G(13)-mediated signaling pathway is required for pressure overload-induced cardiac remodeling and heart failure. Circulation 126:1972–1982. doi:10.1161/CIRCULATIONAHA.112.109256
Vaidya D, Tamaddon HS, Lo CW, Taffet SM, Delmar M, Morley GE, Jalife J (2001) Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ Res 88:1196–1202
Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De Bock M, Decrock E, Bol M, Vinken M, Rogiers V, Tavernier J, Evans WH, Naus CC, Bukauskas FF, Sipido KR, Heusch G, Schulz R, Bultynck G, Leybaert L (2013) Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol 108:309. doi:10.1007/s00395-012-0309-x
Yeager M, Gilula NB (1992) Membrane topology and quaternary structure of cardiac gap junction ion channels. J Mol Biol 223:929–948
Acknowledgments
This work was supported by Grants of the German Research Foundation [Wi270/32-1 and SFB 645, B2 to K.W.; FL 276/3-3 to B.K.F. and P.S.], the National Institutes of Health [R01 HL106632-01, R01 GM57691-13 to M.D.; R01NS072238, RO1HL084464 to F.F.B., as well as NIH HL084583, HL083422 to P J M], the Leducq Foundation to M.D., as well as the American Heart Association and the Saving Tiny Hearts Society to P.J.M.I.L. gratefully acknowledges a PhD stipend of the Jürgen-Manchot-Foundation. We thank Melanie Jokwitz and Christine Siegmund for excellent technical assistance and blastocyst injections.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Delmar and K. Willecke contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 3 (MPG 366 kb)
Rights and permissions
About this article
Cite this article
Lübkemeier, I., Requardt, R.P., Lin, X. et al. Deletion of the last five C-terminal amino acid residues of connexin43 leads to lethal ventricular arrhythmias in mice without affecting coupling via gap junction channels. Basic Res Cardiol 108, 348 (2013). https://doi.org/10.1007/s00395-013-0348-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-013-0348-y