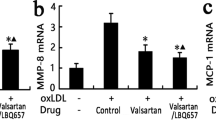
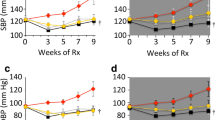
Abstract
Accumulation of biglycan, a small leucine-rich proteoglycan, in the neointima precedes the retention of lipids and accumulation of macrophages during early atherosclerosis. Biglycan is therefore considered a pro-atherogenic proteoglycan that might play a key role in atherogenesis. On the other hand biglycan ensures in part establishment of stable collagen networks. Aim of the present study was to determine whether telmisartan affects biglycan accumulation in a murine model of accelerated atherosclerosis and whether collagen matrix is affected. ApoE−/−-mice on Western diet were chronically (12 weeks) treated either with telmisartan (10 mg/kg) or hydralazine (500 mg/l drinking water) and systolic arterial blood pressure was determined by tail cuff plethysmography. Animals were killed and aortic plaque score, plaque morphology and extracellular matrix as well as cellular plaque composition were analyzed at the aortic root. Furthermore, expression of biglycan and enzymes involved in collagen cross-linking were analyzed in the aorta. Telmisartan and hydralazine lowered systolic arterial blood pressure to the same extent. Biglycan accumulation in the aorta and the aortic root was significantly reduced by telmisartan but not by hydralazine. The amount of collagen and collagen fibril density, macrophages and SMCs was not affected by either treatment as determined by analysis of picrosirius red staining, mac2 and α-SM-actin. Furthermore, telmisartan inhibited aortic plaque score and aortic root plaque size compared to mice receiving hydralazine and untreated controls. The current study shows that telmisartan reduces biglycan accumulation and inhibits atherosclerosis independently of blood pressure lowering and without affecting the collagenous plaque matrix. Thus, biglycan is a pleiotropic target of telmisartan that might contribute to the anti-atherogenic effects of this AT1-antagonist.







Similar content being viewed by others
References
Ahmed MS, Oie E, Vinge LE, Yndestad A, Andersen GG, Andersson Y, Attramadal T, Attramadal H (2003) Induction of myocardial biglycan in heart failure in rats—an extracellular matrix component targeted by AT(1) receptor antagonism. Cardiovasc Res 60:557–568
Ameye L, Aria D, Jepsen K, Oldberg A, Xu T, Young MF (2002) Abnormal collagen fibrils in tendons of biglycan/fibromodulin-deficient mice lead to gait impairment, ectopic ossification, and osteoarthritis. FASEB J 16:673–680
Blessing E, Preusch M, Kranzhofer R, Kinscherf R, Marx N, Rosenfeld ME, Isermann B, Weber CM, Kreuzer J, Grafe J, Katus HA, Bea F (2008) Anti-atherosclerotic properties of telmisartan in advanced atherosclerotic lesions in apolipoprotein E deficient mice. Atherosclerosis 199:295–303
Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV (1997) Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 136:729–743
Grothusen C, Bley S, Selle T, Luchtefeld M, Grote K, Tietge UJ, Drexler H, Schieffer B (2005) Combined effects of HMG-CoA-reductase inhibition and renin–angiotensin system blockade on experimental atherosclerosis. Atherosclerosis 182:57–69
Heegaard AM, Corsi A, Danielsen CC, Nielsen KL, Jorgensen HL, Riminucci M, Young MF, Bianco P (2007) Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation 115:2731–2738
Huang F, Thompson JC, Wilson PG, Aung HH, Rutledge JC, Tannock LR (2008) Angiotensin II increases vascular proteoglycan content preceding and contributing to atherosclerosis development. J Lipid Res 49:521–530
Iozzo RV (1999) The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem 274:18843–18846
Karim MA, Miller DD, Farrar MA, Eleftheriades E, Reddy BH, Breland CM, Samarel AM (1995) Histomorphometric and biochemical correlates of arterial procollagen gene expression during vascular repair after experimental angioplasty. Circulation 91:2049–2057 (see comments)
Kinsella MG, Wight TN (1988) Isolation and characterization of dermatan sulfate proteoglycans synthesized by cultured bovine aortic endothelial cells. J Biol Chem 263:19222–19231
Kunjathoor VV, Chiu DS, O’Brien KD, LeBoeuf RC (2002) Accumulation of biglycan and perlecan, but not versican, in lesions of murine models of atherosclerosis. Arterioscler Thromb Vasc Biol 22:462–468
Little PJ, Osman N, O’Brien KD (2008) Hyperelongated biglycan: the surreptitious initiator of atherosclerosis. Curr Opin Lipidol 19:448–454
Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K (2007) Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arterioscler Thromb Vasc Biol 27:1159–1165
Newby AC, Southgate KM, Davies M (1994) Extracellular matrix degrading metalloproteinases in the pathogenesis of arteriosclerosis. Basic Res Cardiol 89(Suppl 1):59–70
O’Brien KD, Lewis K, Fischer JW, Johnson P, Hwang JY, Knopp EA, Kinsella MG, Barrett PH, Chait A, Wight TN (2004) Smooth muscle cell biglycan overexpression results in increased lipoprotein retention on extracellular matrix: implications for the retention of lipoproteins in atherosclerosis. Atherosclerosis 177:29–35
Ross R (1999) Atherosclerosis is an inflammatory disease. Am Heart J 138:S419–S420
Sasamura H, Shimizu-Hirota R, Nakaya H, Saruta T (2001) Effects of AT1 receptor antagonist on proteoglycan gene expression in hypertensive rats. Hypertens Res 24:165–172
Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, Marsche G, Young MF, Mihalik D, Gotte M, Malle E, Schaefer RM, Grone HJ (2005) The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115:2223–2233
Schaefer L, Beck KF, Raslik I, Walpen S, Mihalik D, Micegova M, Macakova K, Schonherr E, Seidler DG, Varga G, Schaefer RM, Kresse H, Pfeilschifter J (2003) Biglycan, a nitric oxide-regulated gene, affects adhesion, growth, and survival of mesangial cells. J Biol Chem 278:26227–26237
Schönherr E, Järveläinen HT, Kinsella MG, Sandell LJ, Wight TN (1993) Platelet-derived growth factor and transforming growth factor-b1 differentially affect the synthesis of biglycan and decorin by monkey arterial smooth muscle cells. Arterioscler. Thromb. 13:1026–1036
Shimizu-Hirota R, Sasamura H, Mifune M, Nakaya H, Kuroda M, Hayashi M, Saruta T (2001) Regulation of vascular proteoglycan synthesis by angiotensin II type 1 and type 2 receptors. J Am Soc Nephrol 12:2609–2615
Skalen K, Gustafsson M, Rydberg EK, Hulten LM, Wiklund O, Innerarity TL, Boren J (2002) Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417:750–754
Stary HC (1994) Changes in components and structure of atherosclerotic lesions developing from childhood to middle age in coronary arteries. Basic Res Cardiol 89(Suppl 1):17–32
Takaya T, Kawashima S, Shinohara M, Yamashita T, Toh R, Sasaki N, Inoue N, Hirata K, Yokoyama M (2006) Angiotensin II type 1 receptor blocker telmisartan suppresses superoxide production and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. Atherosclerosis 186:402–410
Theocharis AD, Karamanos NK (2002) Decreased biglycan expression and differential decorin localization in human abdominal aortic aneurysms. Atherosclerosis 165:221–230
Tiede K, Stoter K, Petrik C, Chen WB, Ungefroren H, Kruse ML, Stoll M, Unger T, Fischer JW (2003) Angiotensin II AT(1)-receptor induces biglycan in neonatal cardiac fibroblasts via autocrine release of TGFbeta in vitro. Cardiovasc Res 60:538–546
Tran-Lundmark K, Tran PK, Paulsson-Berne G, Friden V, Soininen R, Tryggvason K, Wight TN, Kinsella MG, Boren J, Hedin U (2008) Heparan sulfate in perlecan promotes mouse atherosclerosis: roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ Res 103:43–52
Ungefroren H, Krull NB (1996) Transcriptional regulation of the human biglycan gene. J Biol Chem 271:15787–15795
van Det NF, Tamsma JT, van den Born J, Verhagen NA, van den Heuvel LP, Lowik CW, Berden JH, Bruijn JA, Daha MR, van der Woude FJ (1996) Differential effects of angiotensin II and transforming growth factor beta on the production of heparan sulfate proteoglycan by mesangial cells in vitro. J Am Soc Nephrol 7:1015–1023
Weis SM, Zimmerman SD, Shah M, Covell JW, Omens JH, Ross J Jr, Dalton N, Jones Y, Reed CC, Iozzo RV, McCulloch AD (2005) A role for decorin in the remodeling of myocardial infarction. Matrix Biol 24:313–324
Westermann D, Mersmann J, Melchior A, Freudenberger T, Petrik C, Schaefer L, Lullmann-Rauch R, Lettau O, Jacoby C, Schrader J, Brand-Herrman SM, Young MF, Schultheiss HP, Levkau B, Baba HA, Unger T, Zacharowski K, Tschope C, Fischer JW (2008) Biglycan is required for adaptive remodeling after myocardial infarction. Circulation 117:1269–1276
Wight TN (1995) The extracellular matrix and atherosclerosis. Curr Opin Lipidol 6:326–334
Wight TN, Merrilees MJ (2004) Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ Res 94:1158–1167
Williams KJ, Tabas I (1995) The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15:551–561
Woods A, Couchman JR (1992) Heparan sulfate proteoglycans and signalling in cell adhesion. Adv Exp Med Biol 313:87–96
Xu T, Bianco P, Fisher LW, Longenecker G, Smith E, Goldstein S, Bonadio J, Boskey A, Heegaard AM, Sommer B, Satomura K, Dominguez P, Zhao C, Kulkarni AB, Robey PG, Young MF (1998) Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet 20:78–82
Zernecke A, Weber C (2005) Inflammatory mediators in atherosclerotic vascular disease. Basic Res Cardiol 100:93–101
Zhang C (2008) The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol 103:398–406
Acknowledgments
Financial support by Boehringer Ingelheim Pharma GmbH & Co. KG and scientific contact by Dr. C. Teutsch (Boehringer Ingelheim) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagy, N., Melchior-Becker, A. & Fischer, J.W. Long-term treatment with the AT1-receptor antagonist telmisartan inhibits biglycan accumulation in murine atherosclerosis. Basic Res Cardiol 105, 29–38 (2010). https://doi.org/10.1007/s00395-009-0051-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-009-0051-1