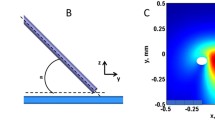
Abstract
Background
The effect of electric stimulation on the polarization of cardiac tissue (virtual electrode effect) is well known; the corresponding response of intracellular calcium concentration ([Ca2+] i ) and its dependence on coupling interval between conditioning stimulus (S1) and test stimulus (S2) has yet to be elucidated.
Objective
Because uncovering the transmembrane potential (V m)–[Ca2+] i relationship during an electric shock is imperative for understanding arrhythmia induction and defibrillation, we aimed to study simultaneous V m and [Ca2+] i responses to strong unipolar stimulation.
Methods
We used a dual-camera optical system to image concurrently V m and [Ca2+] i responses to unipolar stimulation (20 ms ± 20 mA) in Langendorff-perfused rabbit hearts. RH-237 and Rhod-2 fluorescent dyes were used to measure V m and [Ca2+] i , respectively. The S1–S2 interval ranged from 10 to 170 ms to examine stimulation during the action potential.
Results
The [Ca2+] i deflections were less pronounced than changes in V m for all S1–S2 intervals. For cathodal stimulation, [Ca2+] i at the central virtual cathode region increased with prolongation of S1–S2 interval. For anodal stimulation, [Ca2+] i at the central virtual anode area decreased with shortening of the S1–S2 interval. At very short S1–S2 intervals (10–20 ms), when S2 polarization was superimposed on the S1 action potential upstroke, the [Ca2+] i distribution did not follow V m and produced a more complex pattern. After S2 termination [Ca2+] i exhibited three outcomes in a manner similar to V m: non-propagating response, break stimulation, and make stimulation.
Conclusions
Changes in the [Ca2+] i distribution correlate with the behavior of the V m distribution for S1–S2 coupling intervals longer than 20 ms; at shorter intervals S2 creates more heterogeneous [Ca2+] i distribution in comparison with V m. Stimulation in diastole and at very short coupling intervals caused V m–[Ca2+] i uncoupling at the regions of positive polarization (virtual cathode).







Similar content being viewed by others
Abbreviations
- [Ca2+] i :
-
intracellular calcium concentration
- V m :
-
transmembrane potential
- AP:
-
action potential
- CT:
-
calcium transient
- S1:
-
conditioning stimulus
- S2:
-
testing stimulus
- SR:
-
sarcoplasmic reticulum
- VT:
-
ventricular tachycardia
- VF:
-
ventricular fibrillation
- VC:
-
virtual cathode
- VA:
-
virtual anode
- NCX:
-
Na+/Ca2+ exchanger
- I CaL :
-
current via the L-type Ca2+ channels
- I Na/Ca :
-
current via Na+/Ca2+ exchanger
References
Allingham JS, Smith R, Rayment I (2005) The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol 12:378–379
Ashihara T, Trayanova NA (2004) Asymmetry in membrane responses to electric shocks: insights from bidomain simulations. Biophys J 87:2271–2282
Baxter WT, Mironov SF, Zaitsev AV, Jalife J, Pertsov AM (2001) Visualizing excitation waves inside cardiac muscle using transillumination. Biophys J 80:516–530
Bers DM (1997) Ca transport during contraction and relaxation in mammalian ventricular muscle. Basic Res Cardiol 92(Suppl 1):1–10
Bers DM (2001) Excitation-contraction coupling and cardiac contractile force. Kluwer Academic, Dordrecht
Bers DM, Barry WH, Despa S (2003) Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res 57:897–912
Bray MA, Wikswo JP (2003) Examination of optical depth effects on fluorescence imaging of cardiac propagation. Biophys J 85:4134–4145
Cheek ER, Fast VG (2004) Nonlinear changes of transmembrane potential during electrical shocks: role of membrane electroporation. Circ Res 94:208–214
Cheng DK, Tung L, Sobie EA (1999) Nonuniform responses of transmembrane potential during electric field stimulation of single cardiac cells. Am J Physiol 277:H351–H362
Cheng Y, Li L, Nikolski V, Wallick DW, Efimov IR (2004) Shock-induced arrhythmogenesis is enhanced by 2,3-butanedione monoxime compared with cytochalasin D. Am J Physiol Heart Circ Physiol 286:H310–H318
Choi BR, Salama G (2000) Simultaneous maps of optical action potentials and calcium transients in guinea-pig hearts: mechanisms underlying concordant alternans. J Physiol (Lond) 529:171–188
Chudin E, Goldhaber J, Garfinkel A, Weiss J, Kogan B (1999) Intracellular Ca(2+) dynamics and the stability of ventricular tachycardia. Biophys J 77:2930–2941
Cohen NM, Lederer WJ (1988) Changes in the calcium current of rat heart ventricular myocytes during development. J Physiol 406:115–146
Davies DR, Green AL (1956) The kinetics of reactivation, by oximes, of cholinesterase inhibited by organophosphorus compounds. Biochem J 63:529–535
Ding L, Splinter R, Knisley SB (2001) Quantifying spatial localization of optical mapping using Monte Carlo simulations. IEEE Trans Biomed Eng 48:1098–1107
Dou Y, Arlock P, Arner A (2007) Blebbistatin specifically inhibits actin-myosin interaction in mouse cardiac muscle. Am J Physiol Cell Physiol 293:C1148–C1153
Efimov IR, Sidorov V, Cheng Y, Wollenzier B (1999) Evidence of three-dimensional scroll waves with ribbon-shaped filament as a mechanism of ventricular tachycardia in the isolated rabbit heart. J Cardiovasc Electrophysiol 10:1452–1462
Escobar AL, Ribeiro-Costa R, Villalba-Galea C, Zoghbi ME, Perez CG, Mejia-Alvarez R (2004) Developmental changes of intracellular Ca2+ transients in beating rat hearts. Am J Physiol Heart Circ Physiol 286:H971–H978
Fast VG, Rohr S, Ideker RE (2000) Nonlinear changes of transmembrane potential caused by defibrillation shocks in strands of cultured myocytes. Am J Physiol Heart Circ Physiol 278:H688–H697
Fast VG, Cheek ER, Pollard AE, Ideker RE (2004) Effects of electrical shocks on [Ca2+] i and V m in myocyte cultures. Circ Res 94:1589–1597
Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, Efimov IR (2007) Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm 4:619–626
Girouard SD, Laurita KR, Rosenbaum DS (1996) Unique properties of cardiac action potentials recorded with voltage-sensitive dyes. J Cardiovasc Electrophysiol 7:1024–1038
Gomez JP, Potreau D, Branka JE, Raymond G (1994) Developmental changes in Ca2+ currents from newborn rat cardiomyocytes in primary culture. Pflugers Arch 428:241–249
Gray RA (1999) What exactly are optically recorded “action potentials”? J Cardiovasc Electrophysiol 10:1463–1466
Gray RA, Huelsing DJ, Aguel F, Trayanova NA (2001) Effect of strength and timing of transmembrane current pulses on isolated ventricular myocytes. J Cardiovasc Electrophysiol 12:1129–1137
Gray RA, Iyer A, Bray MA, Wikswo JP (2006) Voltage-calcium state-space dynamics during initiation of reentry. Heart Rhythm 3:247–248
Guo W, Kamiya K, Cheng J, Toyama J (1996) Changes in action potentials and ion currents in long-term cultured neonatal rat ventricular cells. Am J Physiol 271:C93–C102
Holcomb MR, Woods MC, Uzelac I, Wikswo JP, Gilligan JM, Sidorov VY (2008) Dual camera system for multimodal imaging of cardiac electrophysiology and metabolism. Submitted
Holmstedt B (1959) Pharmacology of organophosphorus cholinesterase inhibitors. Pharmacol Rev 11:567–688
Hwang GS, Hayashi H, Tang L, Ogawa M, Hernandez H, Tan AY, Li H, Karagueuzian HS, Weiss JN, Lin SF, Chen PS (2006) Intracellular calcium and vulnerability to fibrillation and defibrillation in Langendorff-perfused rabbit ventricles. Circulation 114:2595–2603
Janks DL, Roth BJ (2002) Averaging over depth during optical mapping of unipolar stimulation. IEEE Trans Biomed Eng 49:1051–1054
Josephson IR, Sanchez-Chapula J, Brown AM (1984) A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ Res 54:144–156
Katz AM (2001) Excitation-contraction coupling: calcium fluxes across the sarcoplasmic reticulum. In: Katz AM (eds) Physiology of the heart. Lippincott Williams & Wilkins, Philadelphia, pp 216–239
Kettlewell S, Walker NL, Cobbe SM, Burton FL, Smith GL (2004) The electrophysiological and mechanical effects of 2,3-butane-dione monoxime and cytochalasin-D in the Langendorff perfused rabbit heart. Exp Physiol 89:163–172
Keung EC, Keung CS, Aronson RS (1982) Passive electrical properties of normal and hypertrophied rat myocardium. Am J Physiol 243:H917–H926
Kleber AG, Riegger CB (1987) Electrical constants of arterially perfused rabbit papillary muscle. J Physiol 385:307–324
Knisley SB (1995) Transmembrane voltage changes during unipolar stimulation of rabbit ventricle. Circ Res 77:1229–1239
Kukushkin NI, Bukauskas FF, Sakson ME, Nasonova VV (1975) Anisotropy of stationary rates and delays in extrasystolic waves in the dog heart. Biofizika 20:687–692
Lang RJ, Paul RJ (1991) Effects of 2,3-butanedione monoxime on whole-cell Ca2+ channel currents in single cells of the guinea-pig taenia caeci. J Physiol 433:1–24
Lee MH, Lin SF, Ohara T, Omichi C, Okuyama Y, Chudin E, Garfinkel A, Weiss JN, Karagueuzian HS, Chen PS (2001) Effects of diacetyl monoxime and cytochalasin D on ventricular fibrillation in swine right ventricles. Am J Physiol Heart Circ Physiol 280:H2689–H2696
Litwin SE, Li J, Bridge JH (1998) Na–Ca exchange and the trigger for sarcoplasmic reticulum Ca release: studies in adult rabbit ventricular myocytes. Biophys J 75:359–371
Maack C, O’Rourke B (2007) Excitation-contraction coupling and mitochondrial energetics. Basic Res Cardiol 102:369–392
Neunlist M, Tung L (1995) Spatial distribution of cardiac transmembrane potentials around an extracellular electrode: dependence on fiber orientation. Biophys J 68:2310–2322
Nuss HB, Marban E (1994) Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. J Physiol 479(Pt 2):265–279
Olivetti G, Anversa P, Loud AV (1980) Morphometric study of early postnatal development in the left and right ventricular myocardium of the rat. II. Tissue composition, capillary growth, and sarcoplasmic alterations. Circ Res 46:503–512
Omichi C, Lamp ST, Lin SF, Yang J, Baher A, Zhou S, Attin M, Lee MH, Karagueuzian HS, Kogan B, Qu Z, Garfinkel A, Chen PS, Weiss JN (2004) Intracellular Ca dynamics in ventricular fibrillation. Am J Physiol Heart Circ Physiol 286:H1836–H1844
Puglisi JL, Yuan W, Bassani JWM, Bers DM (1999) Ca2+ influx through Ca2+ channels in rabbit ventricular myocytes during action potential clamp: influence of temperature. Circ Res 85:7e–16e
Raman V, Pollard AE, Fast VG (2007) Shock-induced changes of Ca(i)2+ and V m in myocyte cultures and computer model: dependence on the timing of shock application. Cardiovasc Res 73:101–110
Ruiz-Meana M, Abellan A, Miro-Casas E, Garcia-Dorado D (2007) Opening of mitochondrial permeability transition pore induces hypercontracture in Ca(2+) overloaded cardiac myocytes. Basic Res Cardiol 102:542–552
Sato D, Shiferaw Y, Garfinkel A, Weiss JN, Qu Z, Karma A (2006) Spatially discordant alternans in cardiac tissue: role of calcium cycling. Circ Res 99:520–527
Schlotthauer K, Bers DM (2000) Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ Res 87:774–780
Sepulveda NG, Roth BJ, Wikswo JP Jr (1989) Current injection into a two-dimensional anisotropic bidomain. Biophys J 55:987–999
Sepulveda NG, Wikswo JP Jr (1987) Electric and magnetic fields from two-dimensional anisotropic bisyncytia. Biophys J 51:557–568
Sharma V, Tung L (2002) Effects of uniform electric fields on intracellular calcium transients in single cardiac cells. Am J Physiol Heart Circ Physiol 282:H72–H79
Sidorov VY, Woods MC, Holcomb MR, Wikswo JP (2005) Optical mapping of calcium distribution reveals make and break excitation modes. Heart Rhythm 2:S216
Sidorov VY, Woods MC, Wikswo JP (2003) Effects of elevated extracellular potassium on the stimulation mechanism of diastolic cardiac tissue. Biophys J 84:3470–3479
Snopko RM, Aromolaran AS, Karko KL, Ramos-Franco J, Blatter LA, Mejia-Alvarez R (2007) Cell culture modifies Ca2+ signaling during excitation-contraction coupling in neonate cardiac myocytes. Cell Calcium 41:13–25
Steele DS, Smith GL (1993) Effects of 2,3-butanedione monoxime on sarcoplasmic reticulum of saponin-treated rat cardiac muscle. Am J Physiol 265:H1493–H1500
Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299:1743–1747
Studer R, Reinecke H, Vetter R, Holtz J, Drexler H (1997) Expression and function of the cardiac Na+/Ca2+ exchanger in postnatal development of the rat, in experimental-induced cardiac hypertrophy and in the failing human heart. Basic Res Cardiol 92(Suppl 1):53–58
ter Keurs HE, Wakayama Y, Sugai Y, Price G, Kagaya Y, Boyden PA, Miura M, Stuyvers BD (2006) Role of sarcomere mechanics and Ca2+ overload in Ca2+ waves and arrhythmias in rat cardiac muscle. Ann NY Acad Sci 1080:248–267
Tibbits GF, Xu L, Sedarat F (2002) Ontogeny of excitation-contraction coupling in the mammalian heart. Comp Biochem Physiol A Mol Integr Physiol 132:691–698
Wahler GM, Dollinger SJ, Smith JM, Flemal KL (1994) Time course of postnatal changes in rat heart action potential and in transient outward current is different. Am J Physiol 267:H1157–H1166
Warren M, Huizar JF, Shvedko AG, Zaitsev AV (2007) Spatiotemporal relationship between intracellular Ca2+ dynamics and wave fragmentation during ventricular fibrillation in isolated blood-perfused pig hearts. Circ Res 101:e90–e101
Wibo M, Bravo G, Godfraind T (1991) Postnatal maturation of excitation-contraction coupling in rat ventricle in relation to the subcellular localization and surface density of 1,4-dihydropyridine and ryanodine receptors. Circ Res 68:662–673
Wikswo JP Jr, Lin SF, Abbas RA (1995) Virtual electrodes in cardiac tissue: a common mechanism for anodal and cathodal stimulation. Biophys J 69:2195–2210
Wu S, Weiss JN, Chou C-C, Attin M, Hayashi H, Lin S-F (2005) Dissociation of membrane potential and intracellular calcium during ventricular fibrillation. J Cardiovasc Electrophysiol 16:186–192
Acknowledgments
This work was supported by the NIH (RO1-HL58241, RO1-HL63267), the American Heart Association (0635037N), and the Vanderbilt Institute for Integrative Biosystems Research and Education. We thank Allison Price and Don Berry for their editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Returned for 1. Revision: 22 January 2008 1. Revision received: 13 May 2008
Returned for 2. Revision: 20 June 2008 2. Revision received: 2 July 2008
Rights and permissions
About this article
Cite this article
Sidorov, V.Y., Holcomb, M.R., Woods, M.C. et al. Effects of unipolar stimulation on voltage and calcium distributions in the isolated rabbit heart. Basic Res Cardiol 103, 537–551 (2008). https://doi.org/10.1007/s00395-008-0740-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-008-0740-1