Summary
Background: Mal d 1, the major apple allergen, cross-reacts with IgE specific for the major birch pollen allergen, Bet v 1, and is responsible for birch pollen related food allergy to apple. Isoforms of Bet v 1 showing minor sequence variations display different binding capacitiy for specific IgE antibodies from allergic patients. Moreover, strain-dependent variation of allergenicity has been reported for apples.
Objective: To investigate the occurence of strain-dependent isoforms of Mal d 1 which may differ in their allergenic potential, to obtain data on structures essential for binding of Mal d 1 to the antibody, and to gain insights into the structures responsible for its IgE cross-reactivity to Bet v 1.
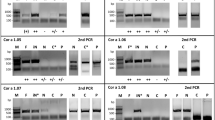
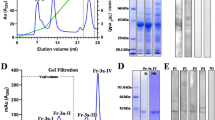
Methods: The cDNA of Mal d 1 from various apple strains was amplified by a PCR strategy based on conserved regions of known Mal d 1-sequences, and sequenced. Two major isoforms of Mal d 1were expressed as recombinant proteins and purified, as were different variants of the major birch pollen allergen, Bet v 1. Together with already existing recombinant birch pollen and apple allergens, these were subjected to allergenicity testing by IgE-immunoblotting, enzyme allergo sorbent test and dose related mediator release. “Hot-spots” for IgE-reactivity were identified by site-directed mutagenesis.
Results: Twelve Mal d 1-clones were sequenced from 7 apple varieties and compared to 3 known Mal d 1 sequences. The clones were clustered into two groups, each showing a high degree of sequence identity to one of the known sequences and specific differences to the third sequence. No strain-specific sequences were identified. In contrast, apple strains with reported differences in allergenicity showed different expression levels of the major allergen. Immunologic testing of recombinant allergens revealed high IgE binding capacity of 2 major isoforms, named GD26 and GS29, with a slightly higher IgE binding capacity of DG26. Moreover, the allergenicity was similar to another rMal d 1 reported in the literature, representing the isoform divergent from our clones. Mutational analysis of our Mal d 1 allergens identified serine in position 111 as essential for IgE binding. Allergenicity was almost depleted by changing this residue into a proline. Moreover, the corresponding serine residue, present in position 112 of Bet v 1, was in a similar manner crucial for the allergenicity of the birch pollen allergen.
Conclusion: We conclude that divergent allergenicity of apple strains mainly depends on differnet expression levels of the major allergen. Introduction of a proline residue in position 111 of Mal d 1 and in position 112 of Bet v 1 led to a drastic reduction of allergenicity of both the pollen and the food allergen, obviously also removing the cross-reactive epitope. Mutants with reduced IgE-reactivity but maintained T-cell reactivity may represent new candidates for a safer specific immunotherapy with reduced side-effects.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 7 June 1999, Accepted: 30 July 1999
Rights and permissions
About this article
Cite this article
Son, D., Scheurer, S., Hoffmann, A. et al. Pollen-related food allergy: cloning and immunological analysis of isoforms and mutants of Mal d 1, the major apple allergen, and Bet v 1, the major birch pollen allergen. Eur J Nutr 38, 201–215 (1999). https://doi.org/10.1007/s003940050063
Issue Date:
DOI: https://doi.org/10.1007/s003940050063