Abstract
Objectives
Intrauterine growth retardation (IUGR) and postnatal nutrition are risk factors for adult metabolic syndrome. However, the influences of long-term high-fat diet (HFD) intake on ectopic fat deposition in non-adipose tissues in IUGR pigs remain unclear. The present study was to determine whether HFD consumption would enhance ectopic fat deposition in IUGR pigs.
Methods
At day 28, IUGR and control pigs were fed ad libitum to either a regular diet or a HFD. Lipid store, enzymatic activities and mRNA expression of lipid metabolism-related factors in liver and semitendinosus muscle (SM) were quantified at postnatal day 178.
Results
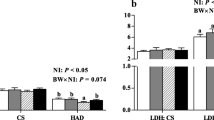
Feeding a HFD to IUGR pigs but not to control pigs significantly increased daily weight gain, carcass fat mass, plasma leptin level and lipid content and lipoprotein lipase (LPL) activity and mRNA abundances of LPL and peroxisome proliferator-activated receptor gamma (PPARγ) in liver and SM, but decreased daily feed intake and mRNA expression of hormone-sensitive lipase (LIPE) and carnitine palmitoyl transferase-1 (CPT-1) in liver and SM (P < 0.05). Compared with control pigs, IUGR pigs had a lower body weight but higher plasma levels of total cholesterol (TC) and insulin (P < 0.05). HFD-fed pigs exhibited greater body weight, plasma concentrations of triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), regardless of birth weight (P < 0.05).
Conclusion
Our results suggested that IUGR increased the vulnerability of HFD-fed pigs to ectopic fat deposition via enhanced fatty acid flux toward ectopic sites and reduced lipolysis and fatty acid oxidation.

Similar content being viewed by others
References
Vernarelli JA, Mitchell DC, Rolls BJ, Hartman TJ (2015) Dietary energy density is associated with obesity and other biomarkers of chronic disease in US adults. Eur J Nutr 54:59–65
Hegarty BD, Cooney GJ, Kraegen EW, Furler SM (2002) Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes 51:1477–1484
Cameron-Smith D, Burke LM, Angus DJ, Tunstall RJ, Cox GR, Bonen A, Hawley JA, Hargreaves M (2003) A short-term, high-fat diet up-regulates lipid metabolism and gene expression in human skeletal muscle. Am J Clin Nutr 77:313–318
Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA (2010) A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr 46:212
Wardle J, Carnell S, Haworth CM, Plomin R (2008) Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. Am J Clin Nutr 87:398–404
Hawkins SS, Cole TJ, Law C (2009) An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK Millennium Cohort Study. J Epidemiol Community Health 63:147–155
Shulman GI (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106:171
Snel M, Jonker JT, Schoones J, Lamb H, de Roos A, Pijl H, Smit J, Meinders A, Jazet I (2012) Ectopic fat and insulin resistance: pathophysiology and effect of diet and lifestyle interventions. Int J Endocrinol. doi:10.1155/2012/983814
Scifres CM, Nelson DM (2009) Intrauterine growth restriction, human placental development and trophoblast cell death. J Physiol 587:3453–3458
Salam RA, Das JK, Bhutta ZA (2014) Impact of intrauterine growth restriction on long-term health. Curr Opin Clin Nutr 17:249–254
Godfrey K, Cameron I, Hanson M (2006) Long-term consequences of foetal restriction. Curr Opin Obstet Gyn 16:267–272
Riddle ES, Campbell MS, Lang BY, Bierer R, Wang Y, Bagley HN, Joss-Moore LA (2014) Intrauterine growth restriction increases TNFα and activates the unfolded protein response in male rat pups. J Obes. doi:10.1155/2014/829862
Hajer GR, van Haeften TW, Visseren FL (2008) Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29:2959–2971
Lane RH, Kelley DE, Ritov VH, Tsirka AE, Gruetzmacher EM (2001) Altered expression and function of mitochondrial β-oxidation enzymes in juvenile intrauterine-growth-retarded rat skeletal muscle. Pediatr Res 50:83–90
Liu J, Chen D, Yao Y, Yu B, Mao X, He J, Huang Z, Zheng P (2012) Intrauterine growth retardation increases the susceptibility of pigs to high-fat diet-induced mitochondrial dysfunction in skeletal muscle. PLoS ONE 7:e34835
Nebendahl C, Krüger R, Görs S, Albrecht E, Martens K, Hennig S, Storm N, Höppner W, Pfuhl R, Metzler-Zebeli BU (2013) Effects on transcriptional regulation and lipid droplet characteristics in the liver of female juvenile pigs after early postnatal feed restriction and refeeding are dependent on birth weight. PLoS ONE 8:e76705
Gondret F, Lefaucheur L, Juin H, Louveau I, Lebret B (2006) Low birth weight is associated with enlarged muscle fiber area and impaired meat tenderness of the longissimus muscle in pigs. J Anim Sci 84:93–103
Wiedmeier JE, Joss-Moore LA, Lane RH, Neu J (2011) Early postnatal nutrition and programming of the preterm neonate. Nutr Rev 69:76–82
Su Y-M, Lv G-R, Xie J-X, Wang Z-H, Lin H-T (2013) Maternal hypoxia increases the susceptibility of adult rat male offspring to high-fat diet-induced nonalcoholic fatty liver disease. Endocrinology 154:4377–4387
Rueda-Clausen CF, Dolinsky VW, Morton JS, Proctor SD, Dyck JR, Davidge ST (2011) Hypoxia-Induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes 60:507–516
Nobili V, Alisi A, Panera N, Agostoni C (2008) Low birth weight and catch-up-growth associated with metabolic syndrome: a 10 year systematic review. Pediatr Endocrinol Rev 6:241–247
Selak MA, Storey BT, Peterside I, Simmons RA (2003) Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol-Endocrinol Metab 285:E130–E137
Lane RH, Kelley DE, Gruetzmacher EM, Devaskar SU (2001) Uteroplacental insufficiency alters hepatic fatty acid-metabolizing enzymes in juvenile and adult rats. Am J Physiol-Regul Integr Comp Physiol 280:R183–R190
Jun H, Daiwen C, Bing Y (2010) Metabolic and transcriptomic responses of weaned pigs induced by different dietary amylose and amylopectin ratio. PLoS ONE 5:e15110. doi:10.1371/journal.pone.0015110
Jiang Z, Zhong W, Zheng C, Lin Y, Yang L, Jiang S (2010) Conjugated linoleic acid differentially regulates fat deposition in backfat and longissimus muscle of finishing pigs. J Anim Sci 88:1694–1705
Sukhija PS, Palmquist D (1988) Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J Agric Food Chem 36:1202–1206
Li FJ, Lin X, Lin SM, Chen WY, Guan Y (2015) Effects of dietary fish oil substitution with linseed oil on growth, muscle fatty acid and metabolism of tilapia (Oreochromis niloticus). Aquac Nutr. doi:10.1111/anu.12270
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29:e45. doi:10.1093/nar/29.9.e45
Liu J, He J, Yu J, Mao X, Zheng P, Huang Z, Yu B, Chen D (2014) Birth weight alters the response to postnatal high-fat diet-induced changes in meat quality traits and skeletal muscle proteome of pigs. Br J Nutr 111:1738–1747
Wang T, Huo YJ, Shi F, Xu RJ, Hutz RJ (2005) Effects of intrauterine growth retardation on development of the gastrointestinal tract in neonatal pigs. Neonatology 88:66–72
Liu C, Lin G, Wang X, Wang T, Wu G, Li D, Wang J (2012) Intrauterine growth restriction alters the hepatic proteome in fetal pigs. J Nutr Biochem 24:954–959
Delahaye F, Breton C, Risold P-Y, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D (2008) Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology 149:470–475
Ramsay T (2005) Porcine preadipocyte proliferation and differentiation: a role for leptin? J Anim Sci 83:2066–2074
Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH (2009) Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes 58:1116–1125
Crume TL, Scherzinger A, Stamm E, McDuffie R, Bischoff KJ, Hamman RF, Dabelea D (2014) The Long-term impact of intrauterine growth restriction in a diverse US cohort of children: the EPOCH study. Obesity 22:608–615
Thompson NM, Norman AM, Donkin SS, Shankar RR, Vickers MH, Miles JL, Breier BH (2007) Prenatal and postnatal pathways to obesity: different underlying mechanisms, different metabolic outcomes. Endocrinology 148:2345–2354
Simmons RA (2007) Developmental origins of diabetes: the role of epigenetic mechanisms. Curr Opin Endocrinol 14:13–16
Koklu E, Kurtoglu S, Akcakus M, Koklu S, Buyukkayhan D, Gumus H, Yikilmaz A (2006) Increased aortic intima-media thickness is related to lipid profile in newborns with intrauterine growth restriction. Horm Res Paediatr 65:269–275
Nüsken K-D, Dötsch J, Rauh M, Rascher W, Schneider H (2008) Uteroplacental insufficiency after bilateral uterine artery ligation in the rat: impact on postnatal glucose and lipid metabolism and evidence for metabolic programming of the offspring by sham operation. Endocrinology 149:1056–1063
Zinkhan EK, Chin JR, Zalla JM, Yu B, Numpang B, Yu X, Jiang C, Callaway CW, McKnight RA, Joss-Moore L (2014) Combination of intrauterine growth restriction and a high-fat diet impairs cholesterol elimination in rats. Pediatr Res 76:432–440
Bekris LM, Galloway NM, Montine TJ, Schellenberg GD, Yu CE (2010) APOE mRNA and protein expression in postmortem brain are modulated by an extended haplotype structure. Am J Med Genet B 153:409–417
Tikkanen MJ, Huttunen J, Ehnholm C, Pietinen P (1990) Apolipoprotein E4 homozygosity predisposes to serum cholesterol elevation during high fat diet. Arterioscler Thromb Vasc 10:285–288
Kim KH (1997) Regulation of mammalian acetyl-coenzyme A carboxylase. Annu Rev Nutr 17:77–99
Mashima T, Seimiya H, Tsuruo T (2009) De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer 100:1369–1372
Dodson MV, Hausman GJ, Guan L, Du M, Rasmussen TP, Poulos SP, Mir P, Bergen WG, Fernyhough ME, McFarland DC (2010) Lipid metabolism, adipocyte depot physiology and utilization of meat animals as experimental models for metabolic research. Int J Biol Sci 6:691
Wang J, Zhao S, Song X, Pan H, Li W, Zhang Y, Gao S, Chen D (2012) Low protein diet up-regulate intramuscular lipogenic gene expression and down-regulate lipolytic gene expression in growth–finishing pigs. Livest Sci 148:119–128
Acknowledgments
This study was financially supported by the earmarked fund for the China Agriculture Research System (CARS-36).
Author’s contributions
D. C., B. Y., J. Y., X. M., P. Z., Z. H. and J. H. contributed to the experimental design and data interpretation and helped in drafting the manuscript. H. Y. carried out the study. H. Y. and P. Z. were responsible for the writing of the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Honglin Yan, Ping Zheng, Bing Yu, Jie Yu, Xiangbing Mao, Jun He, Zhiqing Huang and Daiwen Chen declare no conflict of interest.
Additional information
Honglin Yan and Ping Zheng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yan, H., Zheng, P., Yu, B. et al. Postnatal high-fat diet enhances ectopic fat deposition in pigs with intrauterine growth retardation. Eur J Nutr 56, 483–490 (2017). https://doi.org/10.1007/s00394-015-1093-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-1093-9