Abstract
Purpose
Blueberries are a rich source of anthocyanins (ACNs) and phenolic acids (PA), which are hypothesized to protect against development of atherosclerosis. The present study examined the effect of an ACN- and PA-rich fractions, obtained from a wild blueberry powder, on the capacity to counteract lipid accumulation in macrophages derived from monocytic THP-1 cells. In addition, we tested the capacity of pure ACNs and their metabolites to alter lipid accumulation.
Methods
THP-1-derived macrophages were incubated with fatty acids (500 μM oleic/palmitic acid, 2:1 ratio) and different concentrations (from 0.05 to 10 μg mL−1) of ACN- and PA-rich fractions, pure ACN standards (malvidin, delphinidin and cyanidin 3-glucoside), and metabolites (syringic, gallic and protocatechuic acids). Lipid accumulation was quantified with the fluorescent dye Nile red.
Results
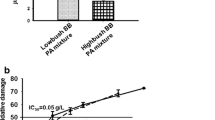
Lipid accumulation was reduced at all concentrations of the ACN-rich fraction tested with a maximum reduction at 10 μg mL−1 (−27.4 %; p < 0.0001). The PA-rich fraction significantly reduced the lipid accumulation only at the low concentrations from 0.05 µg mL−1 to 0.3 µg mL−1, with respect to the control with fatty acids. Supplementation with pure ACN compounds (malvidin and delphinidin-3-glucoside and its metabolic products (syringic and gallic acid)) reduced lipid accumulation especially at the low concentrations, while no significant effect was observed after cyanidin-3-glucoside and protocatechuic acid supplementation.
Conclusions
The results demonstrated a potential role of both the ACN- and PA-rich fractions and single compounds in the lipid accumulation also at concentrations close to that achievable in vivo.






Similar content being viewed by others
References
Nicoué EE, Savard S, Belkacemi K (2007) Anthocyanins in wild blueberries of Quebec: extraction and identification. J Agric Food Chem 55:5626–5635
Veitch NC, Grayer RJ (2011) Flavonoids and their glycosides, including anthocyanins. Nat Prod Rep 28:1626–1695
Andersen OM, Jordheim M (2006) In: Andersen OM, Markham KR (eds) Flavonoids chemistry, biochemistry and applications. CRC Press, Taylor and Francis, Boca Raton, pp 471–551
McGhie TK, Walton MC (2007) The bioavailability and absorption of anthocyanins: towards a better understanding. Mol Nutr Food Res 51:702–713
Tsuda T (2012) Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res 56:159–170
Speciale A, Cimino F, Saija A, Canali R, Virgili F (2014) Bioavailability and molecular activities of anthocyanins as modulators of endothelial function. Genes Nutr 9:404
Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, Chowienczyk P, Jiang B, Cecelja M, Spector T, Macgregor A, Cassidy A (2012) Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am J Clin Nutr 96:781–788
Miguel MG (2011) Anthocyanins: antioxidants and/or anti-inflammatory activities. JAPS 1:07–15
Wu T, Tang Q, Gao Z, Yu Z, Song H, Zheng X, Chen W (2013) Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. Plos One 8:e77585
Chang JJ, Hsu MJ, Huang HP, Chung DJ, Chang YC, Wang CJ (2013) Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J Agric Food Chem 61:6069–6076
Valenti L, Riso P, Mazzocchi A, Porrini M, Fargion S, Agostoni C (2013) Dietary anthocyanins as nutritional therapy for non alcoholic fatty liver disease. Oxid Med Cell Longev 2013:145421
Jia Y, Hoang MH, Jun HJ, Lee JH, Lee SJ (2013) Cyanidin, a natural flavonoid, is an agonistic ligand for liver X receptor alpha and beta and reduces cellular lipid accumulation in macrophages and hepatocytes. Bioorg Med Chem Lett 23:4185–4190
Packard RR, Libby P (2008) Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem 54:24–38
Chen J, Uto T, Tanigawa S, Kumamoto T, Fujii M, Hou DX (2008) Expression profiling of genes targeted by bilberry (Vaccinium myrtillus) in macrophages through DNA microarray. Nutr Cancer 60(Suppl 1):43–50
Mauray A, Felgines C, Morand C, Mazur A, Scalbert A, Milenkovic D (2010) Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo E-deficient mice. Genes Nutr 5:343–353
Wu X, Kang J, Xie C, Burris R, Ferguson ME, Badger TM, Nagarajan S (2010) Dietary blueberries attenuate atherosclerosis in apolipoprotein E-deficient mice by upregulating antioxidant enzyme expression. J Nutr 140:1628–1632
Zanotti I, Dall’Asta M, Mena P, Mele L, Bruni R, Ray S, Del Rio D (2014) Atheroprotective effects of (poly) phenols: a focus on cell cholesterol metabolism. Food Funct. doi:10.1039/c4fo00670d
Manach C, Williamson G, Morand C, Scalbert A, Rémésv C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81(1 Suppl):230S–242S
Faria A, Fernandes I, Norberto S, Mateus N, Calhau C (2014) Interplay between anthocyanins and gut microbiota. J Agric Food Chem 62:6898–6902
Czank C, Cassidy A, Zhang Q, Morrison DJ, Preston T, Kroon PA, Botting NP, Kay CD (2013) Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a13C-tracer study. Am J Clin Nutr 97:995–1003
Kay DC, Kroon PA, Cassidy A (2009) The bioactivity of dietary anthocyanins is likely to be mediated by their degradation products. Mol Nutr Food Res 53:S92–S101
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Rodriguez-Mateos A, Vauzour D, Krueger CG, Shanmuganayagam D, Reed J, Calani L, Mena P, Del Rio D, Crozier A (2014) Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: an update. Arch Toxicol 88:1803–1853
Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, Williamson G (2004) How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr 80:15–21
Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker SF, Smith DM, Sporns P (2005) Handbook of analytical chemistry: pigments, colorants, flavor, texture and bioactive food components, vol 2. Wiley, New Jersey, pp 473–475
Del Bo’ C, Ciappellano S, Klimi-Zacas D, Martini D, Gardana C, Riso P, Porrini M (2010) Anthocyanins adsorption, metabolism, and distribution from a wild-blueberry-enriched diet (Vaccinium angustifolium) is affected by diet duration in the Sprague-Dawley rat. J Agric Food Chem 58:2494–2497
Taverniti V, Fracassetti D, Del Bo’ C, Lanti C, Minuzzo M, Klimis-Zacas D, Riso P, Guglielmetti S (2014) Immunomodulatory effect of a wild blueberry anthocyanin-rich extract in human Caco-2 intestinal cells. J Agric Food Chem 62:8346–8351
Riso P, Brusamolino A, Moro M, Porrini M (2009) Absorption of bioactive compounds from steamed broccoli and their effect on plasma glutathione S-transferase activity. Int J Food Sci Nutr 60(Suppl 1):56–71
Guarnieri S, Riso P, Porrini M (2007) Orange juice vs vitamin C: effect on hydrogen peroxide-induced DNA damage in mononuclear blood cells. Br J Nutr 97:639–643
Simonetti P, Ciappellano S, Gardana C, Bramati L, Pietta P (2002) Procyanidins from Vitis vinifera seeds: in vivo effects on oxidative stress. J Agric Food Chem 50:6217–6221
AOAC Method 991.43 (1995) Total, insoluble and soluble dietary fiber in food-enzymatic-gravimetric method, MES-TRIS buffer. Official methods of analysis, 16th edn. AOAC International, Gaithersburg
Del Bo’ C, Riso P, Brambilla A, Gardana C, Rizzolo A, Simonetti P, Bertolo G, Klimis-Zacas D, Porrini M (2012) Blanching improves anthocyanin absorption from highbush blueberry (Vaccinium corymbosum L.) purée in healthy human volunteers: a pilot study. J Agric Food Chem 60:9298–9304
Cao Y, Roursgaard M, Kermanizadeh A, Loft S, Møller P (2014) Synergistic effects of zinc oxide nanoparticles and fatty acids on toxicity to Caco-2 cells. Int J Toxicol. doi:10.1177/1091581814560032
Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K (1980) Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer 26:171–176
Traore K, Trush MA, George M, Spannhake EW, Anderson W, Asseffa A (2005) Signal transduction of phorbol 12-myristate 13-acetate (PMA)-induced growth inhibition of human monocytic leukemia THP-1 cells is reactive oxygen dependent. Leuk Res 29:863–879
Johnson AC, Yabu JM, Hanson S, Shah VO, Zager RA (2003) Experimental glomerulopathy alters renal cortical cholesterol, SRB1, ABCA1, and HMG CoA, reductase expression. Am J Pathol 162:283–291
Berridge MV, Herst PM, Tan AS (2005) Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol Annu Rev 11:127–152
Vesterdal LK, Danielsen PH, Folkmann JK, Jespersen LF, Aguilar-Pelaez K, Roursgaard M, Loft S, Møller P (2014) Accumulation of lipids and oxidatively damaged DNA in hepatocytes exposed to particles. Toxicol Appl Pharmacol 274:350–360
Moore KJ, Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145:341–355
Quiňones M, Miguel M, Aleixandre A (2013) Beneficial effects of polyphenols on cardiovascular disease. Pharmacol Res 68:125–131
Wallace TC (2011) Anthocyanins in cardiovascular disease. Adv Nutr 2:1–7
Niculescu LS, Sanda GM, Simionescu N, Sima AV (2014) Bilberries exert an anti-atherosclerotic effect in lipid-loaded macrophages. CEJB 9:268–276
Chang JJ, Hsu MJ, Huang HP, Chung DJ, Chang YC, Wang CJ (2013) Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J Agric Food Chem 61:6069–6076
Hwang YP, Choi JH, Han EH, Kim HG, Wee JH, Jung KO, Jung KH, Kwon KI, Jeong TC, Chung YC, Jeong HG (2011) Purple sweet potato anthocyanins attenuate hepatic lipid accumulation through activating adenosine monophosphate-activated protein kinase in human HepG2 cells and obese mice. Nutr Res 31:896–906
Kim HK, Kim JN, Han SN, Nam JH, Na HN, Ha TJ (2012) Black soybean anthocyanins inhibit adipocyte differentiation in 3T3-L1 cells. Nutr Res 32:770–777
Prior RL, Wu X (2006) Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Rad Res 40:1014–1028
Vendrame S, Daugherty A, Kristo AS, Klimis-Zacas D (2014) Wild blueberry (Vaccinium angustifolium)-enriched diet improves dyslipidaemia and modulates the expression of genes related to lipid metabolism in obese Zucker rats. Br J Nutr 111:194–200
Titta L, Trinei M, Stendardo M, Berniakovich I, Petroni K, Tonelli C, Riso P, Porrini M, Minucci S, Pelicci PG, Rapisarda P, Recupero GR, Giorgio M (2010) Blood orange juice inhibits fat accumulation in mice. Int J Obes (Lond) 34:578–588
Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK (2010) Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol 48:937–943
Guo H, Guo J, Jiang X, Li Z, Ling W (2012) Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: involvement of FoxO1-mediated transcription of adipose tryglyceride lipase. Food Chem Toxicol 50:3040–3047
Guo H, Liu G, Zhong R, Wang Y, Wang D, Xia M (2012) Cyanidin-3-O-β-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis 11:10
Tsuda T, Horio F, Uchida K, Aoki H, Osawa T (2003) Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr 133:2125–2130
Speciale A, Chirafisi J, Saija A, Cimino F (2011) Nutritional antioxidants and adaptive cell responses: an update. Curr Mol Med 11:770–789
Chen J, Tao X, Zhang M, Sun A, Zhao L (2014) Properties and stability of blueberry anthocyanin–bovine serum albumin nanoparticles. J Sci Food Agric 94:1781–1786
Cao Y, Roursgaard M, Danielsen PH, Møller P, Loft S (2014) Carbon black nanoparticles promote endothelial activation and lipid accumulation in macrophages independently of intracellular ROS production. PLoS ONE 9:e106711
Cao Y, Jacobsen NR, Danielsen PH, Lenz AG, Stoeger T, Loft S, Wallin H, Roursgaard M, Mikkelsen L, Møller P (2014) Vascular effects of multiwalled carbon nanotubes in dyslipidemic ApoE-/-mice and cultured endothelial cells. Toxicol Sci 138:104–116
Conflict of interest
None of the authors had a personal or financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Del Bo’, C., Cao, Y., Roursgaard, M. et al. Anthocyanins and phenolic acids from a wild blueberry (Vaccinium angustifolium) powder counteract lipid accumulation in THP-1-derived macrophages. Eur J Nutr 55, 171–182 (2016). https://doi.org/10.1007/s00394-015-0835-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-015-0835-z