Abstract
Purpose
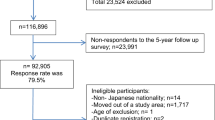
The aim of the study was to assess associations between intake of combined soft drinks (sugar sweetened and artificially sweetened) and fruit and vegetable juices and the risk of hepatocellular carcinoma (HCC), intrahepatic bile duct (IHBC) and biliary tract cancers (GBTC) using data from the European Prospective Investigation into Cancer and Nutrition cohort of 477,206 participants from 10 European countries.
Methods
After 11.4 years of follow-up, 191 HCC, 66 IHBC and 236 GBTC cases were identified. Hazard ratios and 95 % confidence intervals (HR; 95 % CI) were estimated with Cox regression models with multivariable adjustment (baseline total energy intake, alcohol consumption and intake pattern, body mass index, physical activity, level of educational attainment and self-reported diabetes status).
Results
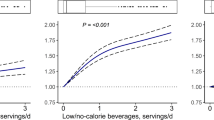
No risk associations were observed for IHBC or GBTC. Combined soft drinks consumption of >6 servings/week was positively associated with HCC risk: HR 1.83; 95 % CI 1.11–3.02, p trend = 0.01 versus non-consumers. In sub-group analyses available for 91 % of the cohort artificially sweetened soft drinks increased HCC risk by 6 % per 1 serving increment (HR 1.06, 95 % CI 1.03–1.09, n cases = 101); for sugar-sweetened soft drinks, this association was null (HR 1.00, 95 % CI 0.95–1.06; n cases = 127, p heterogeneity = 0.07). Juice consumption was not associated with HCC risk, except at very low intakes (<1 serving/week: HR 0.60; 95 % CI 0.38–0.95; p trend = 0.02 vs. non-consumers).
Conclusions
Daily intake of combined soft drinks is positively associated with HCC, but a differential association between sugar and artificially sweetened cannot be discounted. This study provides some insight into possible associations of HCC with sugary drinks intake. Further exploration in other settings is required.

Similar content being viewed by others
Change history
17 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00394-024-03374-2
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- IHBC:
-
Intrahepatic bile duct
- HBV:
-
Hepatitis B
- HCV:
-
Hepatitis C
- T2D:
-
Type 2 diabetes
- NAFLD:
-
Non-alcoholic fatty liver disease
- GBTC:
-
Biliary tract cancer
- EBD:
-
Extrahepatic bile duct cancer
- GB:
-
Gallbladder
- AmpV:
-
Ampulla of Vater
- EPIC:
-
European Prospective Investigation into Cancer and Nutrition
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- GGT:
-
Gamma-glutamyl tranferase
- AP:
-
Liver-specific alkaline phosphatase
- BMI:
-
Body mass index
References
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system, vol 3, 4th edn. IARC, Lyon
IARC (2012) Estimated incidence, mortality and 5-year prevalence. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 27 Aug 2014
WHO (2014) Cancer. http://www.who.int/mediacentre/factsheets/fs297/en/. Accessed 27 Aug 2014
Chuang SC, La Vecchia C, Boffetta P (2009) Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett 286(1):9–14. doi:10.1016/j.canlet.2008.10.040
Trichopoulos D, Bamia C, Lagiou P, Fedirko V, Trepo E, Jenab M, Pischon T, Nothlings U, Overved K, Tjonneland A, Outzen M, Clavel-Chapelon F, Kaaks R, Lukanova A, Boeing H, Aleksandrova K, Benetou V, Zylis D, Palli D, Pala V, Panico S, Tumino R, Sacerdote C, Bueno-De-Mesquita HB, Van Kranen HJ, Peeters PH, Lund E, Quiros JR, Gonzalez CA, Sanchez Perez MJ, Navarro C, Dorronsoro M, Barricarte A, Lindkvist B, Regner S, Werner M, Hallmans G, Khaw KT, Wareham N, Key T, Romieu I, Chuang SC, Murphy N, Boffetta P, Trichopoulou A, Riboli E (2011) Hepatocellular carcinoma risk factors and disease burden in a European cohort: a nested case-control study. J Natl Cancer Inst 103(22):1686–1695. doi:10.1093/jnci/djr395
Blonski W, Kotlyar DS, Forde KA (2010) Non-viral causes of hepatocellular carcinoma. World J Gastroenterol 16(29):3603–3615
Gomaa AI, Khan SA, Toledano MB, Waked I, Taylor-Robinson SD (2008) Hepatocellular carcinoma: epidemiology, risk factors and pathogenesis. World J Gastroenterol 14(27):4300–4308
Schlesinger S, Aleksandrova K, Pischon T, Jenab M, Fedirko V, Trepo E, Overvad K, Roswall N, Tjonneland A, Boutron-Ruault MC, Fagherazzi G, Racine A, Kaaks R, Grote VA, Boeing H, Trichopoulou A, Pantzalis M, Kritikou M, Mattiello A, Sieri S, Sacerdote C, Palli D, Tumino R, Peeters PH, Bueno-de-Mesquita HB, Weiderpass E, Quiros JR, Zamora-Ros R, Sanchez MJ, Arriola L, Ardanaz E, Tormo MJ, Nilsson P, Lindkvist B, Sund M, Rolandsson O, Khaw KT, Wareham N, Travis RC, Riboli E, Nothlings U (2013) Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol 24(9):2449–2455. doi:10.1093/annonc/mdt204
Patel T (2002) Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2:10
Ren HB, Yu T, Liu C, Li YQ (2011) Diabetes mellitus and increased risk of biliary tract cancer: systematic review and meta-analysis. Cancer Causes Control 22(6):837–847. doi:10.1007/s10552-011-9754-3
Fedirko V, Lukanova A, Bamia C, Trichopolou A, Trepo E, Nothlings U, Schlesinger S, Aleksandrova K, Boffetta P, Tjonneland A, Johnsen NF, Overvad K, Fagherazzi G, Racine A, Boutron-Ruault MC, Grote V, Kaaks R, Boeing H, Naska A, Adarakis G, Valanou E, Palli D, Sieri S, Tumino R, Vineis P, Panico S, Bueno-de-Mesquita HB, Siersema PD, Peeters PH, Weiderpass E, Skeie G, Engeset D, Quiros JR, Zamora-Ros R, Sanchez MJ, Amiano P, Huerta JM, Barricarte A, Johansen D, Lindkvist B, Sund M, Werner M, Crowe F, Khaw KT, Ferrari P, Romieu I, Chuang SC, Riboli E, Jenab M (2013) Glycemic index, glycemic load, dietary carbohydrate, and dietary fiber intake and risk of liver and biliary tract cancers in Western Europeans. Ann Oncol 24(2):543–553. doi:10.1093/annonc/mds434
Montonen J, Jarvinen R, Knekt P, Heliovaara M, Reunanen A (2007) Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr 137(6):1447–1454
Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M (2008) Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 22(10):811–816
Abid A, Taha O, Nseir W, Farah R, Grosovski M, Assy N (2009) Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J Hepatol 51(5):918–924. doi:10.1016/j.jhep.2009.05.033
Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB (2010) Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 33(11):2477–2483. doi:10.2337/dc10-1079
Hu FB, Malik VS (2010) Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence. Physiol Behav 100(1):47–54. doi:10.1016/j.physbeh.2010.01.036
Nielsen SJPB (2004) Changes in beverage intake between 1977 and 2001. Am J Prev Med 27(3):205–210
Schernhammer ES, Hu FB, Giovannucci E, Michaud DS, Colditz GA, Stampfer MJ, Fuchs CS (2005) Sugar-sweetened soft drink consumption and risk of pancreatic cancer in two prospective cohorts. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 14(9):2098–2105. doi:10.1158/1055-9965.EPI-05-0059
Bao Y, Stolzenberg-Solomon R, Jiao L, Silverman DT, Subar AF, Park Y, Leitzmann MF, Hollenbeck A, Schatzkin A, Michaud DS (2008) Added sugar and sugar-sweetened foods and beverages and the risk of pancreatic cancer in the National Institutes of Health-AARP Diet and Health Study. Am J Clin Nutr 88(2):431–440
Genkinger JM, Li R, Spiegelman D, Anderson KE, Albanes D, Bergkvist L, Bernstein L, Black A, van den Brandt PA, English DR, Freudenheim JL, Fuchs CS, Giles GG, Giovannucci E, Goldbohm RA, Horn-Ross PL, Jacobs EJ, Koushik A, Mannisto S, Marshall JR, Miller AB, Patel AV, Robien K, Rohan TE, Schairer C, Stolzenberg-Solomon R, Wolk A, Ziegler RG, Smith-Warner SA (2012) Coffee, tea, and sugar-sweetened carbonated soft drink intake and pancreatic cancer risk: a pooled analysis of 14 cohort studies. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 21(2):305–318. doi:10.1158/1055-9965.EPI-11-0945-T
Zhang X, Albanes D, Beeson WL, van den Brandt PA, Buring JE, Flood A, Freudenheim JL, Giovannucci EL, Goldbohm RA, Jaceldo-Siegl K, Jacobs EJ, Krogh V, Larsson SC, Marshall JR, McCullough ML, Miller AB, Robien K, Rohan TE, Schatzkin A, Sieri S, Spiegelman D, Virtamo J, Wolk A, Willett WC, Zhang SM, Smith-Warner SA (2010) Risk of colon cancer and coffee, tea, and sugar-sweetened soft drink intake: pooled analysis of prospective cohort studies. J Natl Cancer Inst 102(11):771–783. doi:10.1093/jnci/djq107
Ren JS, Freedman ND, Kamangar F, Dawsey SM, Hollenbeck AR, Schatzkin A, Abnet CC (2010) Tea, coffee, carbonated soft drinks and upper gastrointestinal tract cancer risk in a large United States prospective cohort study. Eur J Cancer 46(10):1873–1881. doi:10.1016/j.ejca.2010.03.025
Lagergren J, Viklund P, Jansson C (2006) Carbonated soft drinks and risk of esophageal adenocarcinoma: a population-based case-control study. J Natl Cancer Inst 98(16):1158–1161. doi:10.1093/jnci/djj310
Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondiere UR, Hemon B, Casagrande C, Vignat J, Overvad K, Tjonneland A, Clavel-Chapelon F, Thiebaut A, Wahrendorf J, Boeing H, Trichopoulos D, Trichopoulou A, Vineis P, Palli D, Bueno-De-Mesquita HB, Peeters PH, Lund E, Engeset D, Gonzalez CA, Barricarte A, Berglund G, Hallmans G, Day NE, Key TJ, Kaaks R, Saracci R (2002) European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Public Health Nutr 5(6B):1113–1124. doi:10.1079/PHN2002394
Margetts BM, Pietinen P (1997) European prospective investigation into cancer and nutrition: validity studies on dietary assessment methods. Int J Epidemiol 26(Suppl 1):S1–S5
Slimani N, Deharveng G, Unwin I, Southgate DA, Vignat J, Skeie G, Salvini S, Parpinel M, Moller A, Ireland J, Becker W, Farran A, Westenbrink S, Vasilopoulou E, Unwin J, Borgejordet A, Rohrmann S, Church S, Gnagnarella P, Casagrande C, van Bakel M, Niravong M, Boutron-Ruault MC, Stripp C, Tjonneland A, Trichopoulou A, Georga K, Nilsson S, Mattisson I, Ray J, Boeing H, Ocke M, Peeters PH, Jakszyn P, Amiano P, Engeset D, Lund E, de Magistris MS, Sacerdote C, Welch A, Bingham S, Subar AF, Riboli E (2007) The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr 61(9):1037–1056. doi:10.1038/sj.ejcn.1602679
EFSA (2001) COUNCIL DIRECTIVE 2001/112/EC of 20 December 2001 relating to fruit juices and certain similar products intended for human consumption. Off J Eur Commun
Byrne T, Wilmore D (1998) Does growth hormone and glutamine enhance bowel absorption? Gastroenterology 114(5):1110–1112
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65(4 Suppl):1220S–1228S (discussion 1229S–1231S)
Aragon G, Younossi ZM (2010) When and how to evaluate mildly elevated liver enzymes in apparently healthy patients. Clevel Clin J Med 77(3):195–204. doi:10.3949/ccjm.77a.09064
Ventura EE, Davis JN, Goran MI (2011) Sugar content of popular sweetened beverages based on objective laboratory analysis: focus on fructose content. Obesity (Silver Spring) 19(4):868–874. doi:10.1038/oby.2010.255
Densupsoontorn N, Jirapinyo P, Thamonsiri N, Wongarn R, Phosuya P, Tritiprat A, Patraarat S, Pidatcha P, Suwannthol L (2002) Comparison of the nutrient content of fresh fruit juices vs commercial fruit juices. J Med Assoc Thail 85(Suppl 2):S732–S738
Malik VS, Pan A, Willett WC, Hu FB (2013) Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 98(4):1084–1102. doi:10.3945/ajcn.113.058362
Hu FB (2013) Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev 14(8):606–619. doi:10.1111/obr.12040
Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB (2010) Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 121(11):1356–1364. doi:10.1161/CIRCULATIONAHA.109.876185
Ludwig DS (2002) The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 287(18):2414–2423
Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ (2008) Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr 87(5):1194–1203
Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF (2008) Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 48(6):993–999. doi:10.1016/j.jhep.2008.02.011
Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Blendis L, Halpern Z, Oren R (2007) Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): a population based study. J Hepatol 47(5):711–717. doi:10.1016/j.jhep.2007.06.020
Rafiq N. YZM (2008) The impact of non-alcoholic fatty liver disease on chronic hepatitis B and C. Pract Gastroenterol (July 2008) (Viral hepatitis, Series 7)
Romaguera D, Norat T, Wark PA, Vergnaud AC, Schulze MB, van Woudenbergh GJ, Drogan D, Amiano P, Molina-Montes E, Sanchez MJ, Balkau B, Barricarte A, Beulens JW, Clavel-Chapelon F, Crispim SP, Fagherazzi G, Franks PW, Grote VA, Huybrechts I, Key TJ, Khaw KT, Nilsson P, Overvad K, Palli D, Panico S, Quiros JR, Rolandsson O, Sacerdote C, Sieri S, Slimani N, Spijkerman AM, Tjonneland A, Tormo MJ, Tumino R, van den Berg SW, Wermeling PR, Zamara-Ros R, Feskens EJ, Langenberg C, Sharp SJ, Foroughi NG, Riboli E, Wareham NJ (2013) Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia 56(7):1520–1530. doi:10.1007/s00125-013-2899-8
Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A, Kuperman Y, Harmelin A, Kolodkin-Gal I, Shapiro H, Halpern Z, Segal E, Elinav E (2014) Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514(7521):181–186. doi:10.1038/nature13793
Duffey KJ, Popkin BM (2006) Adults with healthier dietary patterns have healthier beverage patterns. J Nutr 136(11):2901–2907
Bazzano LA, Li TY, Joshipura KJ, Hu FB (2008) Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care 31(7):1311–1317. doi:10.2337/dc08-0080
Potter JD, Steinmetz K (1996) Vegetables, fruit and phytoestrogens as preventive agents. IARC Sci Publ 139:61–90
Stagos D, Amoutzias GD, Matakos A, Spyrou A, Tsatsakis AM, Kouretas D (2012) Chemoprevention of liver cancer by plant polyphenols. Food Chem Toxicol 50(6):2155–2170. doi:10.1016/j.fct.2012.04.002
Cust AE, Skilton MR, van Bakel MM, Halkjaer J, Olsen A, Agnoli C, Psaltopoulou T, Buurma E, Sonestedt E, Chirlaque MD, Rinaldi S, Tjonneland A, Jensen MK, Clavel-Chapelon F, Boutron-Ruault MC, Kaaks R, Nothlings U, Chloptsios Y, Zylis D, Mattiello A, Caini S, Ocke MC, van der Schouw YT, Skeie G, Parr CL, Molina-Montes E, Manjer J, Johansson I, McTaggart A, Key TJ, Bingham S, Riboli E, Slimani N (2009) Total dietary carbohydrate, sugar, starch and fibre intakes in the European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr 63(Suppl 4):S37–S60. doi:10.1038/ejcn.2009.74
Acknowledgments
The authors’ responsibilities were as follows—ER: is the overall PI of the EPIC study which is jointly coordinated from ICL and IARC; MS, VF and MJ: conceptualised, designed, obtained funding for and carried out the present research; MS: performed the statistical analysis; and MS, VF, and MJ: contributed to the writing of the manuscript and data interpretation. Contributing authors from each collaborating centres provided the original data and biological samples, information on the respective populations, advice on study design/analysis, and interpretation of the results and approval of the final version of the manuscript for publication. This work was supported by the French National Cancer Institute (L’Institut National du Cancer; INCA) (Grant Number 2009-139). The coordination of EPIC is financially supported by the European Commission (DG-SANCO); and the International Agency for Research on Cancer. The national cohorts are supported by Danish Cancer Society (Denmark); Ligue Contre le Cancer; Institut Gustave Roussy; Mutuelle Générale de l’Education Nationale; and Institut National de la Santé et de la Recherche Médicale (INSERM) (France); Deutsche Krebshilfe, Deutsches Krebsforschungszentrum (DKFZ); and Federal Ministry of Education and Research (Germany); Stavros Niarchos Foundation; Hellenic Health Foundation; and Ministry of Health and Social Solidarity (Greece); Italian Association for Research on Cancer (AIRC); National Research Council; and AIRE-ONLUS Ragusa, AVIS Ragusa, Sicilian Government (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS); Netherlands Cancer Registry (NKR); LK Research Funds; Dutch Prevention Funds; Dutch ZON (Zorg Onderzoek Nederland); World Cancer Research Fund (WCRF); and Statistics Netherlands (the Netherlands); European Research Council (ERC) (Grant Number ERC-2009-AdG 232997) and Nordforsk; and Nordic Center of Excellence Programme on Food, Nutrition and Health (Norway); Health Research Fund (FIS); Regional Governments of Andalucía, Asturias, Basque Country, Murcia (No. 6236) and Navarra; and ISCIII RETIC (RD06/0020) and the Catalan Institute of Oncology. (Spain); Swedish Cancer Society; Swedish Scientific Council; and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK; Medical Research Council; Stroke Association; British Heart Foundation; Department of Health; Food Standards Agency; and Wellcome Trust (UK). Reagents for the hepatitis infection determinations were kindly provided by Abbott Diagnostics Division, Lyon, France. The funding sources had no influence on the design of the study; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Conflict of interest
None of the authors had a conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stepien, M., Duarte-Salles, T., Fedirko, V. et al. Consumption of soft drinks and juices and risk of liver and biliary tract cancers in a European cohort. Eur J Nutr 55, 7–20 (2016). https://doi.org/10.1007/s00394-014-0818-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0818-5