Abstract
Purpose
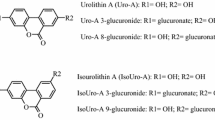
Urolithins, gut microbiota metabolites derived from ellagic acid and ellagitannins, reach micromolar concentrations in the colon lumen where can have anti-inflammatory and anticancer effects. The antiproliferative activity of urolithins (Uro-A, Uro-B, Uro-C and Uro-D) and their most relevant in vivo glucuronides were evaluated in three human colon cancer cell lines (Caco-2, SW480 and HT-29).
Methods
Cell proliferation was evaluated by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide and Trypan blue exclusion assays. Cell cycle was evaluated by flow cytometry and urolithins metabolism by HPLC–MS/MS.
Results
Urolithins inhibited cell proliferation and cell cycle progression in a time- and dose-dependent manner and arrested the cells at S and G2/M phases, depending on the urolithin. Uro-A exerted the highest antiproliferative activity, followed by Uro-C, Uro-D and Uro-B. Unlike Caco-2 and SW480 cells, HT-29 cells partially overcame the effects after 48 h, which was related to the complete glucuronidation of urolithins. Uro-A or Uro-B glucuronides did not affect cell cycle and showed lower antiproliferative activity than their aglycone counterparts. Uro-A or Uro-B plus inhibitors of drug efflux ABC transporters partially prevented the glucuronidation of urolithins in HT-29 cells which became more sensitive.
Conclusions
Uro-A, Uro-B, Uro-C and Uro-D exerted different antiproliferative effects depending on the colon cancer cell line. We also report here, for the first time, the role of ABC transporters and Phase-II metabolism in HT-29 cells as a mechanism of cancer resistance against urolithins due to their conversion to glucuronide conjugates that exerted lower antiproliferative activity.





Similar content being viewed by others
Abbreviations
- ABC:
-
ATP-binding cassette
- ACN:
-
Acetonitrile
- ATP:
-
Adenosine-5′-triphosphate
- BCRP:
-
Breast cancer resistance protein
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- DNA:
-
Deoxyribonucleic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- ESI:
-
Electrospray interface
- Glur:
-
Glucuronide
- HPLC:
-
High-performance liquid chromatography
- IT:
-
Ion trap
- MDCKII:
-
Mardin–Darby canine kidney
- MEM:
-
Minimal essential medium
- MeOH:
-
Methanol
- MRP:
-
Multidrug resistant protein
- MS:
-
Mass spectrometry
- MTT:
-
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- OH-:
-
Hydroxyl groups
- PBS:
-
Phosphate-buffered saline
- P-gp:
-
P-glycoprotein
- RNA:
-
Ribonucleic acid
- SD:
-
Standard deviation
- TNF-α:
-
Tumor necrosis factor alpha
- UGTs:
-
UDP-glucuronosyltransferases
- Uro:
-
Urolithins
- UV:
-
Ultraviolet
- μM:
-
Micromolar
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Thomasset SC, Berry DP, Garcea G, Marczylo T, Steward WP, Gescher AJ (2006) Dietary polyphenolic phytochemicals-promising cancer chemopreventive agents in humans? A review of their clinical properties. Int J Cancer 120:451–458
Rudolf E, Andelová H, Cervinka M (2007) Polyphenolic compounds in chemoprevention of colon cancer-targets and signalling pathways. Anticancer Agents Med Chem 7:559–575
Pan MH, Lai CS, Wu JC, Ho CT (2011) Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res 55:32–45
Clifford MN, Scalbert A (2000) Ellagitannins-nature, occurrence and dietary burden. J Sci Food Agric 80:118–125
Cerdá B, Periago PM, Espín JC, Tomás-Barberán FA (2005) Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. J Agric Food Chem 53:5571–5576
Larrosa M, García-Conesa MT, Espín JC, Tomás-Barberán FA (2010) Ellagitannins, ellagic acid and vascular health. Mol Asp Med 31:513–539
Espín JC, González-Barrio R, Cerdá B, López-Bote C, Rey AI, Tomás-Barberán FA (2007) Iberian pig as a model to clarify obscure points in the bioavailability and metabolism of ellagitannins in humans. J Agric Food Chem 55:10476–10485
González-Sarrías A, Azorín-Ortuño M, Yáñez-Gascón MJ, Tomás-Barberán FA, García-Conesa MT, Espín JC (2009) Dissimilar in vitro and in vivo effects of ellagic acid and its microbiota-derived metabolites, urolithins, on the cytochrome P450 1A1. J Agric Food Chem 57:5623–5632
González-Barrio R, Truchado P, Ito H, Espín JC, Tomás-Barberán FA (2011) UV and MS identification of urolithins and nasutins, the bioavailable metabolites of ellagitannins and ellagic acid in different mammals. J Agric Food Chem 59:1152–1162
González-Sarrías A, Giménez-Bastida JA, García-Conesa MT, Gómez-Sánchez MB, García-Talavera NV, Gil-Izquierdo A, Sánchez-Alvarez C, Fontana-Compiano LO, Morga-Egea JP, Pastor-Quirante FA, Martínez-Díaz F, Tomás-Barberán FA, Espín JC (2010) Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res 54:311–322
Truchado P, Larrosa M, García-Conesa MT, Cerdá B, Vidal-Guevara ML, Tomás-Barberán FA, Espín JC (2012) Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans. J Agric Food Chem 60:5749–5754
Cerdá B, Espín JC, Parra S, Martínez P, Tomás-Barberán FA (2004) The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivates by the colonic microflora of healthy humans. Eur J Nutr 43:205–220
Seeram NP, Aronson WJ, Zhang Y, Henning SM, Moro A, Lee RP, Sartippour M, Harris DM, Rettig M, Suchard MA, Pantuck AJ, Belldegrun A, Heber D (2007) Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem 55:7732–7737
González-Sarrías A, Espín JC, Tomás-Barberán FA, García-Conesa MT (2009) Gene expression, cell cycle arrest and MAPK signalling regulation in Caco-2 cells exposed to ellagic acid and its metabolites, urolithins. Mol Nutr Food Res 53:686–698
Sharma M, Li L, Celver J, Killian C, Kovoor A, Seeram NP (2010) Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J Agric Food Chem 58:3965–3969
Kasimsetty SG, Bialonska D, Reddy MK, Ma G, Khan SI, Ferreira D (2010) Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J Agric Food Chem 58:2180–2187
Larrosa M, González-Sarrías A, Yáñez-Gascón MJ, Selma MV, Azorín-Ortuño M, Toti S, Tomás-Barberán F, Dolara P, Espín JC (2010) Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem 21:717–725
González-Sarrías A, Larrosa M, Tomás-Barberán FA, Dolara P, Espín JC (2010) NF-kappaB-dependent anti-inflammatory activity of urolithins, gut microbiota ellagic acid-derived metabolites, in human colonic fibroblasts. Br J Nutr 104:503–512
Verzelloni E, Pellacani C, Tagliazucchi D, Tagliaferri S, Calani L, Costa LG, Brighenti F, Borges G, Crozier A, Conte A, Del Rio D (2011) Antiglycative and neuroprotective activity of colon-derived polyphenol catabolites. Mol Nutr Food Res 55:S35–S43
Giménez-Bastida JA, Larrosa M, González-Sarrías A, Tomás-Barberán F, Espín JC, García-Conesa MT (2012) Intestinal ellagitannin metabolites ameliorate cytokine-induced inflammation and associated molecular markers in human colon fibroblasts. J Agric Food Chem 60:8866–8876
Dell’agli M, Galli GV, Bulgari M, Basilico N, Romeo S, Bhattacharya D, Taramelli D, Bosisio E (2010) Ellagitannins of the fruit rind of pomegranate (Punica granatum) antagonize in vitro the host inflammatory response mechanisms involved in the onset of malaria. Malar J 9:208
Giménez-Bastida JA, Truchado P, Larrosa M, Espín JC, Tomás-Barberán FA, Allende A, García-Conesa MT (2012) Urolithins, ellagitannin metabolites produced by colon microbiota, inhibit quorum sensing in Yersinia enterocolitica: phenotypic response and associated molecular changes. Food Chem 132:1465–1474
Larrosa M, González-Sarrías A, García-Conesa MT, Tomás-Barberán FA, Espín JC (2006) Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities. J Agric Food Chem 54:1611–1620
Giménez-Bastida JA, González-Sarrías A, Larrosa M, Tomás-Barberán F, Espín JC, García-Conesa MT (2012) Ellagitannin metabolites, urolithin A glucuronide and its aglycone urolithin A, ameliorate TNF-α-induced inflammation and associated molecular markers in human aortic endothelial cells. Mol Nutr Food Res 56:784–796
González-Sarrías A, Miguel V, Merino G, Lucas R, Morales JC, Tomás-Barberán F, Álvarez AI, Espín JC (2013) The gut microbiota ellagic acid-derived metabolite urolithin A, and its sulfate conjugate, are substrates for the drug efflux transporter breast cancer resistance protein (ABCG2/BCRP). J Agric Food Chem 61:4352–4359
Huang Y, Sadée W (2006) Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett 239:168–182
Morgan DM (1998) Tetrazolium (MTT) assay for cellular viability and activity. Methods Mol Biol 79:179–183
Zhang L, Zuo Z, Lin G (2007) Intestinal and hepatic glucuronidation of flavonoids. Mol Pharmacol 4:833–845
Shen SC, Chen YC, Hsu FL, Lee WR (2003) Differential apoptosis-inducing effect of quercetin and its glycosides in human promyeloleukemic HL-60 cells by alternative activation of the caspase 3 cascade. J Cell Biochem 89:1044–1055
Aires V, Limagne E, Cotte AK, Latruffe N, Ghiringhelli F, Delmas D (2013) Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol Nutr Food Res 57:1170–1181
Riddick DS, Lee C, Ramji S, Chinje EC, Cowen RL, Williams KJ, Patterson AV, Stratford IJ, Morrow CS, Townsend AJ, Jounaidi Y, Chen CS, Su T, Lu H, Schwartz PS, Waxman DJ (2005) Cancer chemotherapy and drug metabolism. Drug Metab Dispos 33:1083–1096
Glavinas H, Krajcsi P, Cserepes J, Sarkadi B (2004) The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv 1:27–42
Glavinas H, Kis E, Pál A, Kovács R, Jani M, Vági E, Molnár E, Bánsághi S, Kele Z, Janáky T, Báthori G, von Richter O, Koomen GJ, Krajcsi P (2007) ABCG2 (breast cancer resistance protein/mitoxantrone resistance-associated protein) ATPase assay: a useful tool to detect drug-transporter interactions. Drug Metab Dispos 35:1533–1542
Fukuda Y, Schuetz JD (2012) ABC transporters and their role in nucleoside and nucleotide drug resistance. Biochem Pharmacol 83:1073–1083
Espín JC, Larrosa M, García-Conesa MT, Tomás-Barberán, FA (2013) Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med 270418
Stolarczyk M, Piwowarski JP, Granica S, Stefańska J, Naruszewicz M, Kiss AK (2013) Extracts from Epilobium sp. herbs, their components and gut microbiota metabolites of Epilobium Ellagitannins, Urolithins, inhibit hormone-dependent prostate cancer cells-(LNCaP) proliferation and PSA secretion. Phytother Res. doi:10.1002/ptr.4941
Larrosa M, Tomás-Barberán FA, Espín JC (2004) The grape and wine polyphenol piceatannol is a potent inducer of apoptosis in human SK-Mel-28 melanoma cells. Eur J Nutr 43:275–284
Larrosa M, Tomás-Barberán FA, Espín JC (2006) The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem 17:611–625
Cummings J, Ethell BT, Jardine L, Boyd G, Macpherson JS, Burchell B, Smyth JF, Jodrell DI (2003) Glucuronidation as a mechanism of intrinsic drug resistance in human colon cancer: reversal of resistance by food additives. Cancer Res 63:8443–8450
Gagnon JF, Bernard O, Villeneuve L, Têtu B, Guillemette C (2006) Irinotecan inactivation is modulated by epigenetic silencing of UGT1A1 in colon cancer. Clin Cancer Res 12:1850–1858
Zhang H, Tolonen A, Rousu T, Hirvonen J, Finel M (2011) Effects of cell differentiation and assay conditions on the UDP-glucuronosyltransferase activity in Caco-2 cells. Drug Metab Dispos 39:456–464
Sugatani J, Osabe M, Kurosawa M, Kitamura N, Ikari A, Miwa M (2010) Induction of UGT1A1 and CYP2B6 by an antimitogenic factor in HepG2 cells is mediated through suppression of cyclin-dependent kinase 2 activity: cell cycle-dependent expression. Drug Metab Dispos 38:177–186
Álvarez AI, Vallejo F, Barrera B, Merino G, Prieto JG, Tomás-Barberán FA, Espín JC (2011) Bioavailability of the glucuronide and sulfate conjugates of genistein and daidzein in breast cancer resistance protein 1 knockout mice. Drug Metab Dispos 39:2008–2012
Acknowledgments
This work was funded by the Projects CICYT AGL2011-22447 (MINECO, Spain), Consolider Ingenio 2010 (CSD2007-00063, Fun-C-Food), and Fundación Seneca de la Región de Murcia, Spain (Grupo de Excelencia GERM 06 04486 and 05556/PI/04).
Conflict of interest
Authors declare no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
González-Sarrías, A., Giménez-Bastida, J.A., Núñez-Sánchez, M.Á. et al. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur J Nutr 53, 853–864 (2014). https://doi.org/10.1007/s00394-013-0589-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0589-4