Abstract
Purpose
Recently, several large, randomized clinical trials have proven the benefits of eicosapentaenoic acid (EPA) in cardiovascular prevention. However, the precise protective mechanisms of EPA for heart disease are still controversial. In this study, we evaluate the possible protective effects of EPA, especially the role of autophagy, against cardiomyocyte apoptosis induced by oxidative stress.
Methods
H9C2 myocardioblasts were incubated with 80 μM EPA for 24 h and then exposed to 400 μM of hydrogen peroxide for 3 h. Autophagic response, lysosome function, and apoptosis were analyzed at the end of the experiment.
Results
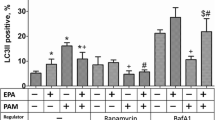
Preincubation of EPA significantly inhibited apoptosis and increased cell viability for H9C2 cells under oxidative stress. The effects of EPA on apoptosis and cell viability were suppressed by 3-MA treatment (autophagic inhibitor). Oxidative stress decreased Beclin 1 protein expression, increased the ratio of LC3II/LC3I, and reduced the formation of acid organelles, whereas the preincubation of EPA abrogated the negative effect of oxidative stress on H9C2 by mediating the autophagic response. Inhibiting autophagy by 3-methyladenine reversed the EPA effect significantly by increasing the ratio of LC3II–LC3I. Treatment with 3-MA did not alter the increment of acid organelles by EPA preincubation. In addition, EPA restored the phosphorylation of Akt activated by H2O2 treatment and induced the phosphorylation of AMPK in H9C2 cells under oxidative stress.
Conclusion
EPA attenuated oxidative stress-induced cardiomyocyte apoptosis by activating an adaptive autophagic response.




Similar content being viewed by others
References
Takemura G, Maruyama R, Goto K, Kanamori H, Tsujimoto A, Minatoguchi S, Fujiwara H (2009) Fate of isolated adult cardiomyocytes undergoing starvation-induced autophagic degeneration. Autophagy 5(1):90–92
Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC (2012) Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS One 7(3):e32388. doi:10.1371/journal.pone.0032388
Takagi H, Matsui Y, Sadoshima J (2007) The role of autophagy in mediating cell survival and death during ischemia and reperfusion in the heart. Antioxid Redox Signal 9(9):1373–1381
Takagi H, Matsui Y, Hirotani S, Sakoda H, Asano T, Sadoshima J (2007) AMPK mediates autophagy during myocardial ischemia in vivo. Autophagy 3(4):405–407
Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA (2011) Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci USA 108(10):4123–4128. doi:10.1073/pnas.1015081108
Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13(5):619–624. doi:10.1038/nm1574
Cetrullo S, Tantini B, Flamigni F, Pazzini C, Facchini A, Stefanelli C, Caldarera CM, Pignatti C (2012) Antiapoptotic and antiautophagic effects of eicosapentaenoic acid in cardiac myoblasts exposed to palmitic acid. Nutrients 4(2):78–90. doi:10.3390/nu4020078
Duda MK, O’Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC (2009) Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res 81(2):319–327
Jung UJ, Torrejon C, Tighe AP, Deckelbaum RJ (2008) n-3 Fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. Am J Clin Nutr 87(6):2003S–2009S
Ogita H, Node K, Asanuma H, Sanada S, Takashima S, Minamino T, Soma M, Kim J, Hori M, Kitakaze M (2003) Eicosapentaenoic acid reduces myocardial injury induced by ischemia and reperfusion in rabbit hearts. J Cardiovasc Pharmacol 41(6):964–969
Engelbrecht AM, Engelbrecht P, Genade S, Niesler C, Page C, Smuts M, Lochner A (2005) Long-chain polyunsaturated fatty acids protect the heart against ischemia/reperfusion-induced injury via a MAPK dependent pathway. J Mol Cell Cardiol 39(6):940–954. doi:10.1016/j.yjmcc.2005.08.004
Pignier C, Revenaz C, Rauly-Lestienne I, Cussac D, Delhon A, Gardette J, Le Grand B (2007) Direct protective effects of poly-unsaturated fatty acids, DHA and EPA, against activation of cardiac late sodium current: a mechanism for ischemia selectivity. Basic Res Cardiol 102(6):553–564. doi:10.1007/s00395-007-0676-x
Raimondi L, Lodovici M, Visioli F, Sartiani L, Cioni L, Alfarano C, Banchelli G, Pirisino R, Cecchi E, Cerbai E, Mugelli A (2005) n-3 polyunsaturated fatty acids supplementation decreases asymmetric dimethyl arginine and arachidonate accumulation in aging spontaneously hypertensive rats. Eur J Nutr 44(6):327–333. doi:10.1007/s00394-004-0528-5
Leroy C, Tricot S, Lacour B, Grynberg A (2008) Protective effect of eicosapentaenoic acid on palmitate-induced apoptosis in neonatal cardiomyocytes. Biochim Biophys Acta 1781(11–12):685–693. doi:10.1016/j.bbalip.2008.07.009
Chen CY, Hsu HC, Lee AS, Tang D, Chow LP, Yang CY, Chen H, Lee YT, Chen CH (2012) The most negatively charged low-density lipoprotein L5 induces stress pathways in vascular endothelial cells. J Vasc Res 49(4):329–341. doi:10.1159/000337463
Zhang XQ, Dunner K Jr, Benedict WF (2010) Autophagy is induced by adenoviral-mediated interferon alpha treatment in interferon resistant bladder cancer and normal urothelial cells as a cell death protective mechanism but not by the bystander factors produced. Cancer Gene Ther 17(8):579–584. doi:10.1038/cgt.2010.14
Nemchenko A, Chiong M, Turer A, Lavandero S, Hill JA (2011) Autophagy as a therapeutic target in cardiovascular disease. J Mol Cell Cardiol 51(4):584–593. doi:10.1016/j.yjmcc.2011.06.010
Xie M, Morales CR, Lavandero S, Hill JA (2011) Tuning flux: autophagy as a target of heart disease therapy. Curr Opin Cardiol 26(3):216–222. doi:10.1097/HCO.0b013e328345980a
Ma X, Godar RJ, Liu H, Diwan A (2012) Enhancing lysosome biogenesis attenuates BNIP3-induced cardiomyocyte death. Autophagy 8(3):297–309
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A (2012) Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia–reperfusion injury. Circulation 125(25):3170–3181. doi:10.1161/CIRCULATIONAHA.111.041814
Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM (2010) Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 285(14):10850–10861. doi:10.1074/jbc.M109.080796
Alers S, Loffler AS, Wesselborg S, Stork B (2012) Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol 32(1):2–11. doi:10.1128/MCB.06159-11
Mihaylova MM, Shaw RJ (2011) The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol 13(9):1016–1023. doi:10.1038/ncb2329
Miyamoto S, Murphy AN, Brown JH (2009) Akt mediated mitochondrial protection in the heart: metabolic and survival pathways to the rescue. J Bioenerg Biomembr 41(2):169–180. doi:10.1007/s10863-009-9205-y
Chaanine AH, Hajjar RJ (2011) AKT signalling in the failing heart. Eur J Heart Fail 13(8):825–829. doi:10.1093/eurjhf/hfr080
Horie T, Ono K, Nagao K, Nishi H, Kinoshita M, Kawamura T, Wada H, Shimatsu A, Kita T, Hasegawa K (2008) Oxidative stress induces GLUT4 translocation by activation of PI3-K/Akt and dual AMPK kinase in cardiac myocytes. J Cell Physiol 215(3):733–742. doi:10.1002/jcp.21353
Acknowledgments
This work was supported by the Research Grant NSC 98-2314-B-002-121-MY3, NSC 100-2313-B-002-053, NSC 101-2313-B-002-030-MY3 and 10R70615-3. The study was sponsored, conducted, and analyzed by the National Science Council and National Taiwan University. The authors are grateful to Miss Chein Jun Kao for technical assistance.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsu, HC., Chen, CY., Chiang, CH. et al. Eicosapentaenoic acid attenuated oxidative stress-induced cardiomyoblast apoptosis by activating adaptive autophagy. Eur J Nutr 53, 541–547 (2014). https://doi.org/10.1007/s00394-013-0562-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0562-2