Abstract
Purpose
Luteolin, a flavone present in many foods and medicinal plants, may have beneficial effects on various human chronic diseases. In the present study, we investigated the hypothesis that luteolin can directly act on vascular endothelial cells (ECs), leading to nitric oxide (NO) production and subsequent vascular relaxation.
Methods
Rat aortic rings were mounted in organ bath. Luteolin was added cumulatively, and vessel relaxation of rat aortic rings precontracted with phenylephrine (PE) or potassium was recorded. Endothelial nitric oxide synthase (eNOS) phosphorylation at Ser1177 and NO production from aortic rings and primary human aortic endothelial cells (HAECs) exposed to luteolin were measured by using Western blot and fluorometric assay, respectively.
Results
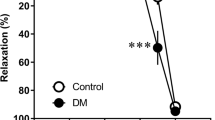
Luteolin dose-dependently (10–100 μmol/L) elicited relaxation of PE- or potassium-contracted aortic rings. The vasorelaxation effect of luteolin was attenuated by the eNOS inhibitor, N-nitro-l-arginine methyl ester, suggesting that this luteolin action is at least partially mediated by activating eNOS activity. We further found that luteolin dose-dependently (10–100 μmol/L) increased eNOS phosphorylation at Ser1177 (up to 1.9-fold) in isolated rat rings. Consistently, exposure of HAECs to luteolin also increased eNOS phosphorylation and NO production.
Conclusions
Luteolin may be a vascular protective agent by directly acting on vascular ECs to stimulate NO-dependent vascular dilatation.






Similar content being viewed by others
Abbreviations
- Ach:
-
Acetylcholine
- α1-AR:
-
α (1) Adrenergic receptor
- [Ca2+]i:
-
Cytosolic Ca2+ levels
- cGMP:
-
Cyclic guanosine monophosphate
- DMSO:
-
Dimethyl sulfoxide
- EGM2:
-
Endothelial growth supplements 2
- eNOS:
-
Endothelial nitric oxide synthase
- FBS:
-
Fetal bovine serum
- HAECs:
-
Human aortic endothelial cells
- HBSS:
-
Hank’s balanced salt solution
- KH:
-
Krebs–Henseleit
- L-NAME:
-
N-nitro-l-arginine methyl ester
- NO:
-
Nitric oxide
- PE:
-
Phenylephrine
- PKA:
-
Protein kinase A
- SMCs:
-
Smooth muscle cells
References
Wang YG, Dedkova EN, Ji X, Blatter LA, Lipsius SL (2005) Phenylephrine acts via IP3-dependent intracellular NO release to stimulate L-type Ca2+ current in cat atrial myocytes. J Physiol 567:143–157
Martin W, Furchgott RF, Villani GM, Jothianandan D (1986) Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther 237:529–538
Schulz E, Jansen T, Wenzel P, Daiber A, Munzel T (2008) Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10:1115–1126
Ribera-Casado JM (1999) Ageing and the cardiovascular system. Z Gerontol Geriatr 32:412–419
Miean KH, Mohamed S (2001) Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J Agric Food Chem 49:3106–3112
Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr 22:19–34
Manach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Chowdhury AR, Sharma S, Mandal S, Goswami A, Mukhopadhyay S, Majumder HK (2002) Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem J 366:653–661
Yee SB, Lee JH, Chung HY, Im KS, Bae SJ, Choi JS, Kim ND (2003) Inhibitory effects of luteolin isolated from Ixeris sonchifolia Hance on the proliferation of HepG2 human hepatocellular carcinoma cells. Arch Pharmacal Res 26:151–156
Lv PC, Li HQ, Xue JY, Shi L, Zhu HL (2008) Synthesis and biological evaluation of novel luteolin derivatives as antibacterial agents. Eur J Med Chem 44:908–914
Shimoi K, Saka N, Kaji K, Nozawa R, Kinae N (2000) Metabolic fate of luteolin and its functional activity at focal site. BioFactors (Oxford, England) 12:181–186
Xagorari A, Papapetropoulos A, Mauromatis A, Economou M, Fotsis T, Roussos C (2001) Luteolin inhibits an endotoxin-stimulated phosphorylation cascade and proinflammatory cytokine production in macrophages. J Pharmacol Exp Ther 296:181–187
Horvathova K, Novotny L, Tothova D, Vachalkova A (2004) Determination of free radical scavenging activity of quercetin, rutin, luteolin and apigenin in H2O2-treated human ML cells K562. Neoplasma 51:395–399
Seelinger G, Merfort I, Schempp CM (2008) Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med 74:1667–1677
Englisch W, Beckers C, Unkauf M, Ruepp M, Zinserling V (2000) Efficacy of Artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung 50:260–265
Gebhardt R (2002) Prevention of taurolithocholate-induced hepatic bile canalicular distortions by HPLC-characterized extracts of artichoke (Cynara scolymus) leaves. Planta Med 68:776–779
Zapolska-Downar D, Zapolski-Downar A, Naruszewicz M, Siennicka A, Krasnodebska B, Koldziej B (2002) Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci 71:2808–2897
Li H, Xia N, Brausch I, Yao Y, Forstermann U (2004) Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J Pharmacol Exp Ther 310:926–932
Rossoni G, Grande S, Galli C, Visioli F (2005) Wild artichoke prevents the age-associated loss of vasomotor function. J Agric Food Chem 53:10291–10296
Ko WC, Shih CM, Leu IJ, Chen TT, Chang JP (2005) Mechanisms of relaxant action of luteolin in isolated guinea pig trachea. Planta Med 71:406–411
Uydes-Dogan BS, Takir S, Ozdemir O, Kolak U, Topcu G, Ulubelen A (2005) The comparison of the relaxant effects of two methoxylated flavones in rat aortic rings. Vascul Pharmacol 43:220–226
Calderone V, Chericoni S, Martinelli C, Testai L, Nardi A, Morelli I, Breschi MC, Martinotti E (2004) Vasorelaxing effects of flavonoids: investigation on the possible involvement of potassium channels. Naunyn Schmiedebergs Arch Pharmacol 370:290–298
Mundy AL, Haas E, Bhattacharya I, Widmer CC, Kretz M, Hofmann-Lehmann R, Minotti R, Barton M (2007) Fat intake modifies vascular responsiveness and receptor expression of vasoconstrictors: implications for diet-induced obesity. Cardiovasc Res 73:368–375
Liu D, Jiang H, Grange RW (2005) Genistein activates the 3′,5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology 146:1312–1320
Liu D, Si H, Reynolds KA, Zhen W, Jia Z, Dillon JS (2007) Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Galphai protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology 148:3068–3076
Kitamura T, Kimura K, Makondo K, Furuya DT, Suzuki M, Yoshida T, Saito M (2003) Proinsulin C-peptide increases nitric oxide production by enhancing mitogen-activated protein-kinase-dependent transcription of endothelial nitric oxide synthase in aortic endothelial cells of Wistar rats. Diabetologia 46:1698–1705
Liu D, Homan LL, Dillon JS (2004) Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5′-monophosphate-dependent mechanism. Endocrinology 145:5532–5539
Collins EM, Walsh MP, Morgan KG (1992) Contraction of single vascular smooth muscle cells by phenylephrine at constant [Ca2+]i. Am J Physiol 262:H754–H762
Karaki H, Urakawa N, Kutsky P (1984) Potassium-induced contraction in smooth muscle. Nihon Heikatsukin Gakkai Zasshi 20:427–444
Peach MJ (1977) Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev 57:313–370
Sharma RV, Bhalla RC (1988) Calcium and abnormal reactivity of vascular smooth muscle in hypertension. Cell Calcium 9:267–274
Furspan PB, Lamb FS, Ross PV, Webb RC, Bohr DF (1986) Calcium and vascular smooth muscle membrane in hypertension. Prog Clin Biol Res 219:225–243
Puscas L, Gilau L, Coltau M, Pasca R, Domuta G, Baican M, Hecht A (2000) Calcium channel blockers reduce blood pressure in part by inhibiting vascular smooth muscle carbonic anhydrase I. Cardiovasc Drugs Ther 14:523–528
Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP (2000) Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol 184:409–420
Lehen’kyi VV, Zelensky SN, Stefanov AV (2005) Ca2+-sensitivity and cGMP-independent effects of NO in vascular smooth muscle. Nitric Oxide 12:105–113
Wu DQ, Lee CH, Rhee SG, Simon MI (1992) Activation of phospholipase C by the alpha subunits of the Gq and G11 proteins in transfected Cos-7 cells. J Biol Chem 267:1811–1817
Wang L, Rolfe M, Proud CG (2003) Ca(2+)-independent protein kinase C activity is required for alpha1-adrenergic-receptor-mediated regulation of ribosomal protein S6 kinases in adult cardiomyocytes. Biochem J 373:603–611
Van Hove CE, Van der Donckt C, Herman AG, Bult H, Fransen P (2009) Vasodilator efficacy of nitric oxide depends on mechanisms of intracellular calcium mobilization in mouse aortic smooth muscle cells. Br J Pharmacol 158:920–930
Xu YC, Leung SW, Yeung DK, Hu LH, Chen GH, Che CM, Man RY (2007) Structure-activity relationships of flavonoids for vascular relaxation in porcine coronary artery. Phytochemistry 68:1179–1188
Si H, Liu D (2008) Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J Nutr 138:297–304
Brossette T, Hundsdorfer C, Kroncke KD, Sies H, Stahl W (2011) Direct evidence that (−)-epicatechin increases nitric oxide levels in human endothelial cells. Eur J Nutr 50:595–599
Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F (2010) (−)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension 55:1398–1405
Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, Hara Y, Yamamoto H, Kinae N (1998) Intestinal absorption of luteolin and luteolin 7-O-beta-glucoside in rats and humans. FEBS Lett 438:220–224
Kim YH, Hwang JH, Noh JR, Gang GT, Kim do H, Son HY, Kwak TH, Shong M, Lee IK, Lee CH (2010) Activation of NAD(P)H:quinone oxidoreductase ameliorates spontaneous hypertension in an animal model via modulation of eNOS activity. Cardiovasc Res 91:519–527
Acknowledgments
We would like to thank the financial supports from the National Center for Complementary and Alternative Medicine of National Institute of Health (1R21AT004694 and 1R21AT002739 to D. L) and American Diabetes Association (1-08-JF-30 to D. Liu).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Si, H., Wyeth, R.P. & Liu, D. The flavonoid luteolin induces nitric oxide production and arterial relaxation. Eur J Nutr 53, 269–275 (2014). https://doi.org/10.1007/s00394-013-0525-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0525-7