Abstract
Purpose
The dietary sesamol is one of the important constituent of sesame seed that has been mainly claimed to combat cardiovascular disease and diabetes, which are the major secondary complications of arthritis. Thus, the present study was designed to evaluate the anti-arthritic, anti-inflammatory and anti-stress potentials of sesamol.
Methods
Arthritis was induced using Freund’s complete adjuvant to hind paw of experimental rats. The physical and biochemical alterations and its recovery by sesamol were assessed by measuring enzymatic and non-enzymatic mediators. Arthritis-induced inflammation, oxidative stress and their protective by sesamol were measured by determining the levels of pro-inflammatory cytokines and oxidative stress markers.
Results
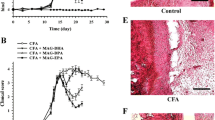
In the present study, sesamol was demonstrated to alleviate arthritis-induced cartilage degeneration by mitigating augmented serum levels of hyaluronidase and matrix metalloproteinases (MMP-13, MMP-3 and MMP-9). It also protected bone resorption by reducing the elevated levels of bone joint exoglycosidases, cathepsin D and tartarate-resistant acid phosphatases. Sesamol also abrogated the non-enzymatic inflammatory markers (TNF, IL-1β, IL-6, COX-2, PGE2, ROS, and H2O2,) effectively. In addition, sesamol neutralizes arthritis-induced oxidative stress by restoring the levels of reactive oxygen species, lipid and hydro peroxides and sustained antioxidant homeostasis by re-establishing altered activities of superoxide dismutase, catalase and glutathione-s-transferase.
Conclusion
Taken together, the study demonstrated the anti-arthritic, anti-inflammatory, anti-oxidative stress and chondro-protective potentials of sesamol in vivo. Thus, sesamol could be a single bullet that can fight arthritis as well as the secondary complications of arthritis such as cardio vascular disorders and diabetes.






Similar content being viewed by others
Abbreviations
- MMPs:
-
Matrix metalloproteinases
- HAases:
-
Hyaluronidases
- ROS:
-
Reactive oxygen species
- DCFDA:
-
Dihydrodichlorofluorescin diacetate
- HVA:
-
Homovanillic acid
- TBST:
-
Tris buffer saline tween
References
Labianca R, Sarzi-Puttini P, Zuccaro SM, Cherubino P, Vellucci R, Fornasari D (2012) Adverse effects associated with non-opioid and opioid treatment in patients with chronic pain. Clin Drug Investig 32(1):53–63
Girish KS, Kemparaju K (2007) The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci 80(21):1921–1943
Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K, Hashimoto K, Roach HI, Olivotto E, Borzì RM, Marcu KB (2011) Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater 24(21):202–220
Nagase H, Kashiwagi M (2003) Aggrecanases and cartilage matrix degradation. Arthritis Res Ther 5:94–103
Yasuda T (2011) Activation of Akt leading to NF-κB up-regulation in chondrocytes stimulated with fibronectin fragment. Biomed Res 32(3):209–215
Chopra K, Tiwari V, Arora V, Kuhad A (2010) Sesamol suppresses neuro-inflammatory cascade in experimental model of diabetic neuropathy. J Pain 11:950–957
Ying Z, Kherada N, Kampfrath T, Mihai G, Simonetti O, Desikan R, Selvendiran K, Sun Q, Ziouzenkova O, Parthasarthy S, Rajagopalan S (2011) A modified sesamol derivative inhibits progression of atherosclerosis. Arterioscler Thromb Vasc Biol 31(3):536–542
Nielen MM, van Sijl AM, Peters MJ, Verheij RA, Schellevis FG, Nurmohamed MT (2012) Cardiovascular disease prevalence in patients with inflammatory arthritis, diabetes mellitus and osteoarthritis: a cross-sectional study in primary care. BMC Musculoskelet Disord 13(1):150
Martel RR, Klicius J (1982) Comparison in rats of the anti-inflammatory and gastric irritant effects of etodolac with several clinically effective anti-inflammatory drugs. Agents Actions 12(3):295–297
Girish KS, Jagadeesha DK, Rajeev KB, Kemparaju K (2002) Snake venom hyaluronidase: an evidence for isoforms and extracellular matrix degradation. Mol Cell Biochem 240(1–2):105–110
Kawai Y, Anno K (1971) Mucopolysaccharide-degrading enzymes from the liver of the squid, Ommastrephes sloani pacificus. I Biochem Biophy Acta 242:428–432
Minkin C (1982) Bone acid phosphatase: tartrate-resistant acid phosphatase as a marker of osteoclast function. Calcif Tissue Int 34:285–290
King EJ, Abul-fadl MA, Walker PG (1951) King-Armstrong phosphatase estimation by the determination of liberated phosphate. J Clin Pathol 4(1):85–91
Folin O, Ciocalteau V (1927) Tyrosine and tryptophan determination in protein. J Bio Chem 73:627–631
Driver AS, Kodavanti PS, Mundy WR (2000) Age related changes in reactive oxygen species production in rat brain homogenates. Neurotoxicol Teratol 22:175–181
Botsoglou N, Taitzoglou I, Zervos I, Botsoglou E, Tsantarliotou M, Chatzopoulou PS (2010) Potential of long-term dietary administration of rosemary in improving the antioxidant status of rat tissues following carbon tetrachloride intoxication. Food Chem Toxicol 48(3):944–950
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478
Mokrasch LC, Teschke EJ (1984) Glutathione content of cultured cells and rodent brain regions: a specific fluorometric assay. Anal Biochem 140(2):506–509
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Vladimir AK, Potapovich AI (1989) Superoxide driven oxidation of quercetin under simple sensitive assay for determination of superoxide dismutase. Biochem Int 19:1117–1124
Aebi H (1984) Catalase Methods Enzymol 1059:121–125
Guthenberg C, Alin P, Mannervik B (1985) Glutathione transferase from rat testis. Methods Enzymol 113:507–510
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement using folin-phenol reagent. J Biol Chem 193:265–275
Kaufmann J, Kielstein V, Kilian S, Stein G, Hein G (2003) Relation between body mass index and radiological progression in patients with rheumatoid arthritis. J Rheumatol 30(11):2350–2355
Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson-Hughes B, Dinarello CA, Rosenberg IH (1994) Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest 93(6):2379–2386
Nagaya H, Ymagata T, Ymagata S, Iyoda K, Ito H, Hasegawa Y, Iwata H (1999) Examination of synovial fluid and serum hyaluronidase activity as a joint marker in rheumatoid arthritis and osteoarthritis patients (by zymography). Ann Rheum Dis 58(3):186–188
Shimizu C, Coutts RD, Healey RM, Kubo T, Hirasawa Y, Amiel D (1997) Method of histomorphometric assessment of glycosaminoglycans in articular cartilage. J Orthop Res 15(5):670–674
Sofat N, Robertson SD, Wait R (2012) Fibronectin III 13–14 domains induce joint damage via Toll-like receptor 4 activation and synergize with interleukin-1 and tumour necrosis factor. J Innate Immun 4(1):69–79
Ortutay Z, Polgar A, Gomor B, Geher P, Lakatos T, Glant TT, Gay RE, Gay S, Pállinger E, Farkas C, Farkas E, Tóthfalusi L, Kocsis K, Falus A, Buzás EI (2003) Synovial fluid exoglycosidases are predictors of rheumatoid arthritis and are effective in cartilage glycosaminoglycan depletion. Arthritis Rheum 48:2163–2172
Popko J, Zalewska A, Gołaszewska Z, Marciniak J, Sierakowski S, Worowski K, Zwierz K (2005) Comparative analysis of hexosaminidase and cathepsin D expression in synovial fluid of patients with rheumatoid arthritis and traumatized joints. Clin Exp Rheumatol 23(5):725–726
Janckila AJ, Neustadt DH, Yam LT (2008) Significance of serum TRACP in rheumatoid arthritis. J Bone Miner Res 23(8):1287–1295
Kumar VS, Kumar DA, Kalaivani K, Gangadharan AC, Raju KV, Thejomoorthy P, Manohar BM, Puvanakrishnan R (2005) Optimization of pulsed electromagnetic field therapy for management of arthritis in rats. Bioelectromagnetics 26(6):431–439
Mirshafiey A, Mohsenzadegan M (2008) The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. J Aller Asth Immunol 4:195–202
Shah R, Raska K Jr, Tiku ML (2005) The presence of molecular markers of in vivo lipid peroxidation in osteoarthritic cartilage: a pathogenic role in osteoarthritis. Arthritis Rheum 52(9):2799–2807
Ishii T, Akagawa M, Naito Y, Handa O, Takagi T, Mori T, Kumazawa S, Yoshikawa T, Nakayama T (2010) Pro-oxidant action of pyrroloquinoline quinone: characterization of protein oxidative modifications. Biosci Biotechnol Biochem 74(3):663–666
Sutipornpalangkul W, Morales NP, Charoencholvanich K, Harnroongroj T (2009) Lipid peroxidation, glutathione, vitamin E, and antioxidant enzymes in synovial fluid from patients with osteoarthritis. Int J Rheum Dis 12(4):324–328
Soszyński M, Bartosz G (1997) Decrease in accessible thiols as an index of oxidative damage to membrane proteins. Free Radic Biol Med 23(3):463–469
Karakoc M, Altindag O, Keles H, Soran N, Selek S (2008) Serum oxidative-antioxidative status in patients with ankylosing spondilitis. Rheumatol Int 27:1131–1134
Sharma S, Sahu D, Das HR, Sharma D (2011) Amelioration of collagen-induced arthritis by Salix nigra bark extract via suppression of pro-inflammatory cytokines and oxidative stress. Food Chem Toxicol 49(12):3395–3406
Olszewska-Słonina DM, Matewski D, Drewa G, Woźniak A, Czajkowski R, Rajewski P, Olszewski KJ, Zegarska B (2010) Oxidative equilibrium in the prophylaxis of degenerative joint changes: an analysis of pre- and postoperative activity of antioxidant enzymes in patients with hip and knee osteoarthritis. Med Sci Monit 16(5):238–245
Vieira AT, Silveira KD, Arruda MC, Fagundes CT, Gonçalves JL, Silva TA, Neves MJ, Menezes MA, Nicoli JR, Teixeira MM, Martins FS (2012) Treatment with Selemax®, a selenium-enriched yeast, ameliorates experimental arthritis in rats and mice. Br J Nutr doi:10.1017/S0007114512000013 [Epub ahead of print]
Acknowledgments
HM thanks University Grant Commission for providing UGC-JRF/SRF (SR No. 212830418, Ref No. 20-6/2008(ii) EU-IV). HM also thank Prof. B. S. Vishwanath, DOS in Biochemistry, University of Mysore, Mysore, Dr. S. Naveen, DFRL, Mysore, Mr. Vilas Hiremath and Mr. Babu for their timely help and support. Authors thank Central instrumentation facility, Institute of excellence project, University of Mysore.
Conflict of interest
All the authors hereby disclose that there is no competing conflict of interest among the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hemshekhar, M., Thushara, R.M., Jnaneshwari, S. et al. Attenuation of adjuvant-induced arthritis by dietary sesamol via modulation of inflammatory mediators, extracellular matrix degrading enzymes and antioxidant status. Eur J Nutr 52, 1787–1799 (2013). https://doi.org/10.1007/s00394-012-0482-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0482-6