Abstract
Purpose
The aim of this study was to determine the effects of an atherogenic diet (AD; 40 % lipid, 1.25 % cholesterol, kcal) on triglyceride (TAG) and cholesterol accumulation in liver and on gene expression of liver X receptor (LXR) and farnesoid X receptor (FXR) and their target genes and to observe if these responses are affected by endurance training.
Methods
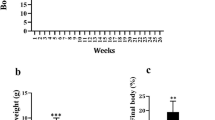
Sprague–Dawley rats (n = 32) were divided into two groups and randomly assigned to an AD or a standard diet (SD) for 7 weeks. Half of the rats in each group were assigned to an exercise training program for 5 days/week.
Results
The AD resulted in a large (P < 0.01) accumulation in liver TAG (4×) along with elevated liver and plasma cholesterol without any gain in peripheral fat mass. The liver TAG and cholesterol accumulations were associated with an important reduction (P < 0.01; 60 %) in FXR, but no change in LXR transcripts. Accompanying the reduction in FXR gene expression, we found an increase (P < 0.001) in SREBP-1c and a decrease (P < 0.01) in MTP mRNAs suggesting an increased lipogenesis and a reduced VLDL production, respectively. The AD was also associated with lower HMG-CoA-r, squalene synthase, and ABCG8 transcripts (P < 0.001). In the intestine, exercise training resulted in higher NPC1L1, ABCG5, and ABCG8 in SD-fed animals, while all these increases were suppressed under the AD feeding.
Conclusions
It is concluded that dietary cholesterol favors liver TAG and cholesterol accumulations associated with an important reduction in FXR transcripts.





Similar content being viewed by others
References
Comhair TM, Garcia Caraballo SC, Dejong CH, Lamers WH, Kohler SE (2011) Dietary cholesterol, female gender and n-3 fatty acid deficiency are more important factors in the development of non-alcoholic fatty liver disease than the saturation index of the fat. Nutr Metab (Lond) 8:4
Treguier M, Briand F, Boubacar A, Andre A, Magot T, Nguyen P, Krempf M, Sulpice T, Ouguerram K (2011) Diet-induced dyslipidemia impairs reverse cholesterol transport in hamsters. Eur J Clin Invest 41(9):921–928
Wang YM, Zhang B, Xue Y, Li ZJ, Wang JF, Xue CH, Yanagita T (2010) The mechanism of dietary cholesterol effects on lipids metabolism in rats. Lipids Health Dis 9:4
Subramanian S, Goodspeed L, Wang S, Kim J, Zeng L, Ioannou GN, Haigh WG, Yeh MM, Kowdley KV, O’Brien KD et al (2011) Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res 52(9):1626–1635
Bhathena J, Kulamarva A, Martoni C, Malgorzata A, Malhotra UM, Paul A, Prakash S (2011) Diet-induced metabolic hamster model of nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes 4:195–203
Wojcicka G, Jamroz-Wisniewska A, Horoszewicz K, Beltowski J (2007) Liver X receptors (LXRs). Part I: structure, function, regulation of activity, and role in lipid metabolism. Postepy Hig Med Dosw (Online) 61:736–759
Redinger RN (2003) Nuclear receptors in cholesterol catabolism: molecular biology of the enterohepatic circulation of bile salts and its role in cholesterol homeostasis. J Lab Clin Med 142(1):7–20
Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ (1996) An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 383(6602):728–731
Cipriani S, Mencarelli A, Palladino G, Fiorucci S (2010) FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res 51(4):771–784
Kong B, Luyendyk JP, Tawfik O, Guo GL (2009) Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther 328(1):116–122
Yang ZX, Shen W, Sun H (2010) Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int 4(4):741–748
Correa Antunez MI, Moran Penco JM, Amaya Lozano JL, Leal Macho A, Macia Botejara E, Saenz Santamaria J (2010) Changes in the fat composition and histomorphology of the liver after partial intestinal resections. Nutr Hosp 25(6):999–1005
Yoshida M (2011) Novel role of NPC1L1 in the regulation of hepatic metabolism: potential contribution of ezetimibe in NAFLD/NASH treatment. Curr Vasc Pharmacol 9(1):121–123
Zhu Y, Li F, Guo GL (2011) Tissue-specific function of farnesoid X receptor in liver and intestine. Pharmacol Res 63(4):259–265
Frith J, Newton JL (2010) Liver disease in older women. Maturitas 65(3):210–214
Gauthier MS, Couturier K, Latour JG, Lavoie JM (2003) Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol 94(6):2127–2134
Meissner M, Havinga R, Boverhof R, Kema I, Groen AK, Kuipers F (2010) Exercise enhances whole-body cholesterol turnover in mice. Med Sci Sports Exerc 42(8):1460–1468
Gollnick PD (1963) Chronic effect of exercise on liver cholesterol of normal and hypercholesteremic rats. Am J Physiol 205:453–456
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226(1):497–509
Xu G, Pan LX, Li H, Forman BM, Erickson SK, Shefer S, Bollineni J, Batta AK, Christie J, Wang TH et al (2002) Regulation of the farnesoid X receptor (FXR) by bile acid flux in rabbits. J Biol Chem 277(52):50491–50496
Fujino T, Murakami K, Ozawa I, Minegishi Y, Kashimura R, Akita T, Saitou S, Atsumi T, Sato T, Ando K et al (2009) Hypoxia downregulates farnesoid X receptor via a hypoxia-inducible factor-independent but p38 mitogen-activated protein kinase-dependent pathway. FEBS J 276(5):1319–1332
Shi QY, Lin YG, Zhou X, Lin YQ, Yan S (2010) Expression of FXR mRNA, PPAR alpha mRNA and bile acid metabolism related genes in intrahepatic cholestasis of pregnant rats. Zhonghua Gan Zang Bing Za Zhi 18(12):927–930
Zhong XY, Yu JH, Zhang WG, Wang ZD, Dong Q, Tai S, Cui YF, Li H (2012) MicroRNA-421 functions as an oncogenic miRNA in biliary tract cancer through down-regulating farnesoid X receptor expression. Gene 493(1):44–51
Fuchs M (2012) Non-alcoholic Fatty liver disease: the bile Acid-activated farnesoid x receptor as an emerging treatment target. J Lipids 2012:934396
Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J (2004) Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest 113(10):1408–1418
Zhang Y, Castellani LW, Sinal CJ, Gonzalez FJ, Edwards PA (2004) Peroxisome proliferator-activated receptor-gamma coactivator 1alpha (PGC-1alpha) regulates triglyceride metabolism by activation of the nuclear receptor FXR. Genes Dev 18(2):157–169
Chapados NA, Lavoie JM (2010) Exercise training increases hepatic endoplasmic reticulum (er) stress protein expression in MTP-inhibited high-fat fed rats. Cell Biochem Funct 28(3):202–210
Kosters A, Frijters RJ, Schaap FG, Vink E, Plosch T, Ottenhoff R, Jirsa M, De Cuyper IM, Kuipers F, Groen AK (2003) Relation between hepatic expression of ATP-binding cassette transporters G5 and G8 and biliary cholesterol secretion in mice. J Hepatol 38(6):710–716
Yu L, Gupta S, Xu F, Liverman AD, Moschetta A, Mangelsdorf DJ, Repa JJ, Hobbs HH, Cohen JC (2005) Expression of ABCG5 and ABCG8 is required for regulation of biliary cholesterol secretion. J Biol Chem 280(10):8742–8747
Lambert G, Amar MJ, Guo G, Brewer HB Jr, Gonzalez FJ, Sinal CJ (2003) The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem 278(4):2563–2570
Gupta S, Pandak WM, Hylemon PB (2002) LXR alpha is the dominant regulator of CYP7A1 transcription. Biochem Biophys Res Commun 293(1):338–343
Ando H, Tsuruoka S, Yamamoto H, Takamura T, Kaneko S, Fujimura A (2005) Regulation of cholesterol 7alpha-hydroxylase mRNA expression in C57BL/6 mice fed an atherogenic diet. Atherosclerosis 178(2):265–269
Pramfalk C, Angelin B, Eriksson M, Parini P (2007) Cholesterol regulates ACAT2 gene expression and enzyme activity in human hepatoma cells. Biochem Biophys Res Commun 364(2):402–409
Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ (2010) Bile acids and their nuclear receptor FXR: relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta 1801(7):683–692
Alvaro A, Rosales R, Masana L, Vallve JC (2010) Polyunsaturated fatty acids down-regulate in vitro expression of the key intestinal cholesterol absorption protein NPC1L1: no effect of monounsaturated nor saturated fatty acids. J Nutr Biochem 21(6):518–525
Levy E, Spahis S, Sinnett D, Peretti N, Maupas-Schwalm F, Delvin E, Lambert M, Lavoie MA (2007) Intestinal cholesterol transport proteins: an update and beyond. Curr Opin Lipidol 18(3):310–318
Altmann SW, Davis HR Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M et al (2004) Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 303(5661):1201–1204
Durstine JL, Grandjean PW, Davis PG, Ferguson MA, Alderson NL, DuBose KD (2001) Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med 31(15):1033–1062
Meissner M, Havinga R, Boverhof R, Kema I, Groen AK, Kuipers F (2010) Exercise enhances whole-body cholesterol turnover in mice. Med Sci Sports Exerc 42(8):1460–1468
Wilund KR, Feeney LA, Tomayko EJ, Weiss EP, Hagberg JM (2009) Effects of endurance exercise training on markers of cholesterol absorption and synthesis. Physiol Res 58(4):545–552
Meissner M, Lombardo E, Havinga R, Tietge UJ, Kuipers F, Groen AK (2011) Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 218(2):323–329
Gauthier MS, Favier R, Lavoie JM (2006) Time course of the development of non-alcoholic hepatic steatosis in response to high-fat diet-induced obesity in rats. Br J Nutr 95(2):273–281
Basciano H, Miller AE, Naples M, Baker C, Kohen R, Xu E, Su Q, Allister EM, Wheeler MB, Adeli K (2009) Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am J Physiol Endocrinol Metab 297(2):E462–E473
Matsuzawa N, Takamura T, Kurita S, Misu H, Ota T, Ando H, Yokoyama M, Honda M, Zen Y, Nakanuma Y et al (2007) Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 46(5):1392–1403
Yiu WF, Kwan PL, Wong CY, Kam TS, Chiu SM, Chan SW, Chan R (2011) Attenuation of fatty liver and prevention of hypercholesterolemia by extract of Curcuma longa through regulating the expression of CYP7A1, LDL-receptor, HO-1, and HMG-CoA reductase. J Food Sci 76(3):H80–H89
Koopmans SJ, Dekker R, Ackermans MT, Sauerwein HP, Serlie MJ, van Beusekom HM, van den Heuvel M, van der Giessen WJ (2011) Dietary saturated fat/cholesterol, but not unsaturated fat or starch, induces C-reactive protein associated early atherosclerosis and ectopic fat deposition in diabetic pigs. Cardiovasc Diabetol 10:64
Zhang Y, Ge X, Heemstra LA, Chen WD, Xu J, Smith JL, Ma H, Kasim N, Edwards PA, Novak CM (2012) Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol Endocrinol 26(2):272–280
Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas A et al (2011) Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 60(7):1861–1871
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR; T 0602 145.02) and the Natural Sciences and Engineering Research Council of Canada (NSERC; 7594).
Conflict of interest
All authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Côté, I., Ngo Sock, E.T., Lévy, É. et al. An atherogenic diet decreases liver FXR gene expression and causes severe hepatic steatosis and hepatic cholesterol accumulation: effect of endurance training. Eur J Nutr 52, 1523–1532 (2013). https://doi.org/10.1007/s00394-012-0459-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0459-5