Abstract
Purpose
Glutamine (Gln) is a nutrient with immunomodulatory effects in metabolic stressed conditions. This study investigated the effects of Gln on colonic-inflammatory-mediator expression and mucosal repair in mice with dextran sulfate sodium (DSS)-induced colitis.
Methods
C57BL/6 mice received distilled water containing 3 % DSS for 5 d to induce colitis. One of the DSS-treated groups was intraperitoneally injected with an alanyl (Ala)-Gln solution 3 days before (G-DSS) while the other group was administered Ala-Gln 3 days after colitis (DSS-G) was induced. The Ala-Gln solution provided 0.5 g Gln/kg/d. The saline-DSS group (S-DSS) received an identical amount of saline before and after colitis was induced to serve as a positive control.
Results
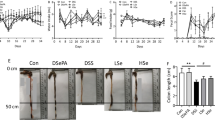
The S-DSS group had a shorter colon length, higher plasma haptoglobin level, and more-severe colon inflammation. Also, the toll-like receptor (TLR)4 level, nuclear factor (NF)-κB activation, and inflammatory cytokine gene expression in the colon were higher than those of the normal control group. Gln administration either before or after colitis suppressed TLR4 protein levels, decreased plasma haptoglobin, and reduced colon inflammation. Histological inflammatory scores were also lowered. Compared to the post-colitis Gln group, preventive use of Gln had higher colon length, expressions of mucin 2, trefoil factor 3, and heat shock protein 72 genes were also upregulated in the colon.
Conclusions
These results suggest that Gln administered either before or after the colitis mitigated inflammation of colitis that was not observed in group without Gln injection. Prophylactic treatment with Gln had more-beneficial effects on reducing inflammatory markers and enhancing the recovery of mucosa in DSS-induced colitis.






Similar content being viewed by others
References
Baumgart DC, Sandborn WJ (2007) Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369:1641–1657
Brown SJ, Mayer L (2007) The immune response in inflammatory bowel disease. Am J Gastroenterol 102:2058–2069
Kelsall BL (2008) Innate and adaptive mechanisms to control pathological intestinal inflammation. J Pathol 214:242–259
Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Zum Buschenfelde KHM (1995) Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD). Clin Exp Immunol 102:448–455
Macpherson A, Khoo UY, Forgacs I, Philpott-Howard J, Bjarnason I (1996) Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut 38:365–375
Palsson-McDermott EM, O’Neill LA (2004) Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113:153–162
Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T et al (2002) Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology 122:1987–2000
Szebeni B, Veres G, Dezsofi A, Rusai K, Vannay A, Mraz M et al (2008) Increased expression of Toll-like receptor (TLR) 2 and TLR4 in the colonic mucosa of children with inflammatory bowel disease. Clin Exp Immunol 151:34–41
Ohkawara T, Takeda H, Miyashita K, Nishiwaki M, Nakayama T, Taniguchi M et al (2006) Regulation of Toll-like receptor 4 expression in mouse colon by macrophage migration inhibitory factor. Histochem Cell Biol 125:575–582
Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H et al (2003) Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol 170:3977–3985
Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG et al (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114:385–391
Shintani N, Nakajima T, Okamoto T, Kondo T, Nakamura N, Mayumi T (1998) Involvement of CD4 + T cells in the development of dextran sulfate sodium-induced experimental colitis and suppressive effect of IgG on their action. Gen Pharmacol 31:477–481
Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW (2000) Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion 62:240–248
Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R et al (2008) Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun 377:12–16
Ito R, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J et al (2006) Interferon-gamma is causatively involved in experimental inflammatory bowel disease in mice. Clin Exp Immunol 146:330–338
Melgar S, Karlsson A, Michaelsson E (2005) Acute colitis induced by dextran sulfate sodium progresses to chronicity in C57BL/6 but not in BALB/c mice: correlation between symptoms and inflammation. Am J Physiol Gastrointest Liver Physiol 288:G1328–G1338
Wang WW, Qiao SY, Li DF (2009) Amino acids and gut function. Amino Acids 37:105–110
Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB (2001) Glutamine reduces cytokine release, organ damage, and mortality in a rat model of endotoxemia. Shock 16:398–402
White JS, Hoper M, Parks RW, Clements WD, Diamond T (2005) Glutamine improves intestinal barrier function in experimental biliary obstruction. Eur Surg Res 37:342–347
Hering NA, Schulzke JD (2009) Therapeutic options to modulate barrier defects in inflammatory bowel disease. Dig Dis 27:450–454
Rao RK, Samak G (2012) Role of glutamine in protection of intestinal epithelial tight junctions. J Epithelial Biol Pharmacol 5:47–54
Belmonte L, Coëffier M, Le Pessot F, Miralles-Barrachina O, Hiron M, Leplingard A et al (2007) Effects of glutamine supplementation on gut barrier, glutathione content and acute phase response in malnourished rats during inflammatory shock. World J Gastroenterol 13:2833–2840
Mok E, Constantin B, Favreau F, Neveux N, Magaud C, Delwail A et al (2008) Hankard R. l-Glutamine administration reduces oxidized glutathione and MAP kinase signaling in dystrophic muscle of mdx mice. Pediatr Res 63:268–273
Iba Y, Sugimoto Y, Kamei C, Masukawa T (2003) Possible role of mucosal mast cells in the recovery process of colitis induced by dextran sulfate sodium in rats. Int Immunopharmacol 3:485–491
Hulsewé KW, van der Hulst RW, van Acker BA, von Meyenfeldt MF, Soeters PB (2004) Inflammation rather than nutritional depletion determines glutamine concentrations and intestinal permeability. Clin Nutr 23:1209–1216
Shiomi Y, Nishiumi S, Ooi M, Hatano N, Shinohara M, Yoshie T et al (2011) GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflamm Bowel Dis 17:2261–2274
Israeli E, Berenshtein E, Wengrower D, Aptekar L, Kohen R, Zajicek G et al (2004) Prophylactic administration of topical glutamine enhances the capability of the rat colon to resist inflammatory damage. Dig Dis Sci 49:1705–1712
Vicario M, Amat C, Rivero M, Moreto M, Pelegri C (2007) Dietary glutamine affects mucosal functions in rats with mild DSS-induced colitis. J Nutr 137:1931–1937
Xue H, Sufit AJ, Wischmeyer PE (2011) Glutamine therapy improves outcome of in vitro and in vivo experimental colitis models. JPEN J Parenter Enteral Nutr 35:188–197
Wang F, Zhao HY, Zhang ST, Gong YZ, Zhang HF, Zhang C (2011) Effect of enteral nutrition on dextran sulfate sodium-induced colitis in rats. J Dig Dis 12:453–458
Kretzmann NA, Fillmann H, Mauriz JL, Marroni CA, Marroni N, Gonzalez-Gallego J et al (2008) Effects of glutamine on proinflammatory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm Bowel Dis 14:1504–1513
Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS et al (2006) Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: role in proliferation and apoptosis in the intestine. Gastroenterology 131:862–877
Ungaro R, Fukata M, Hsu D, Hernandez Y, Breglio K, Chen A et al (2009) A novel Toll-like receptor 4 antagonist antibody ameliorates inflammation but impairs mucosal healing in murine colitis. Am J Physiol Gastrointest Liver Physiol 296:G1167–G1179
Simeonidis S, Stauber D, Chen G, Hendrickson WA, Thanos D (1999) Mechanisms by which IκB proteins control NF-κB activity. Proc Natl Acad Sci USA 96:49–54
Magne N, Toillon RA, Bottero V, Didelot C, Houtte PV, Gerard JP et al (2006) NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett 231:158–168
Khoshnan A, Tindell C, Laux I, Bae D, Bennett B, Nel AE (2000) The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4 + lymphocytes. J Immunol 165:1743–1754
Kolls JK, Linden A (2004) Interleukin-17 family members and inflammation. Immunity 21:467–476
Strieter RM, Kunkel SL, Keane MP, Standiford TJ (1999) Chemokines in lung injury: Thomas A. Neff lecture. Chest 116:103S–110S
Chu CC, Hou YC, Pai MH, Chao CJ, Yeh SL (2011) Pretreatment with alanyl-glutamine suppresses T-helper-cell-associated cytokine expression and reduces inflammatory responses in mice with acute DSS-induced colitis. J Nutr Biochem [Epub ahead of print]
Garrido C, Schmitt E, Cande C, Vahsen N, Parcellier A, Kroemer G (2003) HSP27 and HSP70: potentially oncogenic apoptosis inhibitors. Cell Cycle 2:579–584
Lanneau D, Brunet M, Frisan E, Solary E, Fontenay M, Garrido C (2008) Heat shock proteins: essential proteins for apoptosis regulation. J Cell Mol Med 12:743–761
Sreedhar AS, Csermely P (2004) Heat shock proteins in the regulation of apoptosis: new strategies in tumor therapy: a comprehensive review. Pharmacol Ther 101:227–257
Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB (1997) Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Physiol 272:G879–G884
Shirazi T, Longman RJ, Corfield AP, Probert CS (2000) Mucins and inflammatory bowel disease. Postgrad Med J 76:473–478
Kindon H, Pothoulakis C, Thim L, Lynch-Devaney K, Podolsky DK (1995) Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology 109:516–523
Acknowledgments
This study was supported by a research grant (NSC100-2320-B-038-007-MY3) from the National Science Council, Taipei, Taiwan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, YC., Chu, CC., Ko, TL. et al. Effects of alanyl-glutamine dipeptide on the expression of colon-inflammatory mediators during the recovery phase of colitis induced by dextran sulfate sodium. Eur J Nutr 52, 1089–1098 (2013). https://doi.org/10.1007/s00394-012-0416-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0416-3