Abstract
Background
Multiple cellular stress responses have been implicated in chronic diseases such as obesity, diabetes, cardiovascular, and inflammatory bowel diseases. Even though phenotypically different, chronic diseases share cellular stress signaling pathways, in particular endoplasmic reticulum (ER) unfolded protein response (UPR).
Results and methods
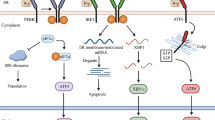
The purpose of the ER UPR is to restore ER homeostasis after challenges of the ER function. Among the triggers of ER UPR are changes in the redox status, elevated protein synthesis, accumulation of unfolded or misfolded proteins, energy deficiency and glucose deprivation, cholesterol depletion, and microbial signals. Numerous mouse models have been used to characterize the contribution of ER UPR to several pathologies, and ER UPR-associated signaling has also been demonstrated to be relevant in humans. Additionally, recent evidence suggests that the ER UPR is interrelated with metabolic and inflammatory pathways, autophagy, apoptosis, and mitochondrial stress signaling. Furthermore, microbial as well as nutrient sensing is integrated into the ER-associated signaling network.
Conclusion
The data discussed in the present review highlight the interaction of ER UPR with inflammatory pathways, metabolic processes and mitochondrial function, and their interrelation in the context of chronic diseases.



Similar content being viewed by others
References
Ogden CL, Lamb MM, Carroll MD, Flegal KM (2010) Obesity and socioeconomic status in children and adolescents: United States, 2005–2008. NCHS Data Brief 2010:1–8
Hossain P, Kawar B, El Nahas M (2007) Obesity and diabetes in the developing world—a growing challenge. N Engl J Med 356:213–215
Sartor RB (2006) Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3:390–407
Rodgers A, Ezzati M, Vander Hoorn S, Lopez AD et al (2004) Distribution of major health risks: findings from the Global Burden of Disease study. PLoS Med 1:e27
Renz H, von Mutius E, Brandtzaeg P, Cookson WO, Autenrieth IB, Haller D (2011) Gene-environment interactions in chronic inflammatory disease. Nat Immunol 12(4):273−277
Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444:860–867
Ma Y, Hendershot LM (2004) The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer 4:966–977
Zhang K, Kaufman RJ (2008) From endoplasmic-reticulum stress to the inflammatory response. Nature 454:455–462
Ozcan U, Cao Q, Yilmaz E, Lee AH et al (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8:519–529
Schroder M, Kaufman RJ (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789
Rutkowski DT, Arnold SM, Miller CN, Wu J et al (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4:e374
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7:880–885
Bertolotti A, Wang X, Novoa I, Jungreis R et al (2001) Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest 107:585–593
Heazlewood CK, Cook MC, Eri R, Price GR et al (2008) Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med 5:e54
Kaser A, Lee AH, Franke A, Glickman JN et al (2008) XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134:743–756
Martinon F, Chen X, Lee AH, Glimcher LH (2010) TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 11:411–418
Deng J, Lu PD, Zhang Y, Scheuner D et al (2004) Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol 24:10161–10168
Rao RV, Ellerby HM, Bredesen DE (2004) Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11:372–380
Hu P, Han Z, Couvillon AD, Kaufman RJ et al (2006) Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol 26:3071–3084
Burkart A, Shi X, Chouinard M, Corvera S (2010) Adenylate kinase 2 links mitochondrial energy metabolism to the induction of the unfolded protein response. J Biol Chem
Pouyssegur J, Shiu RP, Pastan I (1977) Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell 11:941–947
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140:900–917
Scheuner D, Song B, McEwen E, Liu C et al (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7:1165–1176
Pizzo P, Pozzan T (2007) Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol 17:511–517
Lim JH, Lee HJ, Ho Jung M, Song J (2009) Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell Signal 21:169–177
Fukushima K, Fiocchi C (2004) Paradoxical decrease of mitochondrial DNA deletions in epithelial cells of active ulcerative colitis patients. Am J Physiol Gastrointest Liver Physiol 286:G804–G813
Haga N, Saito S, Tsukumo Y, Sakurai J et al. (2010) Mitochondria regulate the unfolded protein response leading to cancer cell survival under glucose deprivation conditions. Cancer Sci
Arduino DM, Esteves AR, Domingues AF, Pereira CM et al (2009) ER-mediated stress induces mitochondrial-dependent caspases activation in NT2 neuron-like cells. BMB Rep 42:719–724
Chen X, Shen J, Prywes R (2002) The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem 277:13045–13052
Calfon M, Zeng H, Urano F, Till JH et al (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415:92–96
Sidrauski C, Walter P (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90:1031–1039
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23:7448–7459
Yamamoto K, Sato T, Matsui T, Sato M et al (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13:365–376
Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH et al (2004) XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21:81–93
Sriburi R, Jackowski S, Mori K, Brewer JW (2004) XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol 167:35–41
Hollien J, Lin JH, Li H, Stevens N et al (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186:323–331
Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K et al (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16:1345–1355
Urano F, Wang X, Bertolotti A, Zhang Y et al (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287:664–666
Yoshida H, Haze K, Yanagi H, Yura T et al (1998) Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273:33741–33749
Kokame K, Kato H, Miyata T (2001) Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J Biol Chem 276:9199–9205
Bommiasamy H, Back SH, Fagone P, Lee K et al (2009) ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci 122:1626–1636
Bailey D, O’Hare P (2007) Transmembrane bZIP transcription factors in ER stress signaling and the unfolded protein response. Antioxid Redox Signal 9:2305–2321
Murakami T, Saito A, Hino S, Kondo S et al (2009) Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol 11:1205–1211
Zhang K, Shen X, Wu J, Sakaki K et al (2006) Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124:587–599
Wu J, Rutkowski DT, Dubois M, Swathirajan J et al (2007) ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13:351–364
Harding HP, Zhang Y, Ron D (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397:271–274
Harding HP, Zhang Y, Bertolotti A, Zeng H et al (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5:897–904
Ma Y, Brewer JW, Diehl JA, Hendershot LM (2002) Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318:1351–1365
Connor JH, Weiser DC, Li S, Hallenbeck JM et al (2001) Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol 21:6841–6850
Brush MH, Weiser DC, Shenolikar S (2003) Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol 23:1292–1303
Cullinan SB, Diehl JA (2006) Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 38:317–332
Cullinan SB, Zhang D, Hannink M, Arvisais E et al (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 23:7198–7209
Garcia MA, Gil J, Ventoso I, Guerra S et al (2006) Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev 70:1032–1060
Nguyen DT, Kebache S, Fazel A, Wong HN et al (2004) Nck-dependent activation of extracellular signal-regulated kinase-1 and regulation of cell survival during endoplasmic reticulum stress. Mol Biol Cell 15:4248–4260
Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL et al (2003) Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol 4:321–329
Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P et al (2001) Plasma cell differentiation requires the transcription factor XBP-1. Nature 412:300–307
Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y et al (2009) Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol 183:1480–1487
Jiang HY, Wek SA, McGrath BC, Scheuner D et al (2003) Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol 23:5651–5663
Li Y, Schwabe RF, DeVries-Seimon T, Yao PM et al (2005) Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem 280:21763–21772
Zhang X, Zhang G, Zhang H, Karin M et al (2008) Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135:61–73
Uehara T, Nakamura T, Yao D, Shi ZQ et al (2006) S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441:513–517
Haynes CM, Titus EA, Cooper AA (2004) Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15:767–776
Woo CW, Cui D, Arellano J, Dorweiler B et al (2009) Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol 11:1473–1480
Messlik A, Schmechel S, Kisling S, Bereswill S et al (2009) Loss of Toll-like receptor 2 and 4 leads to differential induction of endoplasmic reticulum stress and proapoptotic responses in the intestinal epithelium under conditions of chronic inflammation. J Proteome Res 8:4406–4417
Aprahamian CJ, Lorenz RG, Harmon CM, Dimmit RA (2008) Toll-like receptor 2 is protective of ischemia-reperfusion-mediated small-bowel injury in a murine model. Pediatr Crit Care Med 9:105–109
Hoyer-Hansen M, Jaattela M (2007) Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14:1576–1582
Kroemer G, Jaattela M (2005) Lysosomes and autophagy in cell death control. Nat Rev Cancer 5:886–897
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752
Ogata M, Hino S, Saito A, Morikawa K et al (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26:9220–9231
Bernales S, McDonald KL, Walter P (2006) Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol 4:e423
Kouroku Y, Fujita E, Tanida I, Ueno T et al (2007) ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ 14:230–239
Fujita E, Kouroku Y, Isoai A, Kumagai H et al (2007) Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum Mol Genet 16:618–629
Scherz-Shouval R, Elazar Z (2010) Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci 36:30–38
Nakagawa T, Yuan J (2000) Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150:887–894
Nakagawa T, Zhu H, Morishima N, Li E et al (2000) Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403:98–103
Tan Y, Dourdin N, Wu C, De Veyra T et al (2006) Ubiquitous calpains promote caspase-12 and JNK activation during endoplasmic reticulum stress-induced apoptosis. J Biol Chem 281:16016–16024
Morishima N, Nakanishi K, Takenouchi H, Shibata T et al (2002) An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 277:34287–34294
Rao RV, Castro-Obregon S, Frankowski H, Schuler M et al (2002) Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem 277:21836–21842
Fischer H, Koenig U, Eckhart L, Tschachler E (2002) Human caspase 12 has acquired deleterious mutations. Biochem Biophys Res Commun 293:722–726
Hitomi J, Katayama T, Eguchi Y, Kudo T et al (2004) Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol 165:347–356
Fawcett TW, Martindale JL, Guyton KZ, Hai T et al (1999) Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 339(Pt 1):135–141
Lin JH, Li H, Zhang Y, Ron D et al (2009) Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One 4:e4170
Harding HP, Novoa I, Zhang Y, Zeng H et al (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6:1099–1108
Zinszner H, Kuroda M, Wang X, Batchvarova N et al (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12:982–995
McCullough KD, Martindale JL, Klotz LO, Aw TY et al (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21:1249–1259
Puthalakath H, O’Reilly LA, Gunn P, Lee L et al (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129:1337–1349
Marciniak SJ, Yun CY, Oyadomari S, Novoa I et al (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18:3066–3077
Chami M, Oules B, Szabadkai G, Tacine R et al (2008) Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell 32:641–651
Lin JH, Li H, Yasumura D, Cohen HR et al (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318:944–949
Wei MC, Zong WX, Cheng EH, Lindsten T et al (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730
Scorrano L, Oakes SA, Opferman JT, Cheng EH et al (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300:135–139
Zong WX, Li C, Hatzivassiliou G, Lindsten T et al (2003) Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 162:59–69
Hetz C, Bernasconi P, Fisher J, Lee AH et al (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312:572–576
Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M et al (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27:53–66
Wang Y, Vera L, Fischer WH, Montminy M (2009) The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 460:534–537
Back SH, Scheuner D, Han J, Song B et al (2009) Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab 10:13–26
Oyadomari S, Harding HP, Zhang Y, Oyadomari M et al (2008) Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab 7:520–532
Gregor MG, Hotamisligil GS (2007) Adipocyte stress: the endoplasmic reticulum and metabolic disease. J Lipid Res
Chen JC, Wu ML, Huang KC, Lin WW (2008) HMG-CoA reductase inhibitors activate the unfolded protein response and induce cytoprotective GRP78 expression. Cardiovasc Res 80:138–150
Wolins NE, Brasaemle DL, Bickel PE (2006) A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett 580:5484–5491
Lee AH, Scapa EF, Cohen DE, Glimcher LH (2008) Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320:1492–1496
Bobrovnikova-Marjon E, Hatzivassiliou G, Grigoriadou C, Romero M et al (2008) PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc Natl Acad Sci U S A 105:16314–16319
Rutkowski DT, Wu J, Back SH, Callaghan MU et al (2008) UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell 15:829–840
Yang J, Croniger CM, Lekstrom-Himes J, Zhang P et al (2005) Metabolic response of mice to a postnatal ablation of CCAAT/enhancer-binding protein alpha. J Biol Chem 280:38689–38699
Flodby P, Barlow C, Kylefjord H, Ahrlund-Richter L et al (1996) Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein alpha. J Biol Chem 271:24753–24760
Wang ND, Finegold MJ, Bradley A, Ou CN et al (1995) Impaired energy homeostasis in C/EBP alpha knockout mice. Science 269:1108–1112
Nicholas SA, Coughlan K, Yasinska I, Lall GS et al. (2011) Dysfunctional mitochondria contain endogenous high-affinity human Toll-like receptor 4 (TLR4) ligands and induce TLR4-mediated inflammatory reactions. Int J Biochem Cell Biol
Hori O, Ichinoda F, Tamatani T, Yamaguchi A et al (2002) Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J Cell Biol 157:1151–1160
Aldridge JE, Horibe T, Hoogenraad NJ (2007) Discovery of genes activated by the mitochondrial unfolded protein response (mtUPR) and cognate promoter elements. PLoS ONE 2:e874
Zhao Q, Wang J, Levichkin IV, Stasinopoulos S et al (2002) A mitochondrial specific stress response in mammalian cells. EMBO J 21:4411–4419
Horibe T, Hoogenraad NJ (2007) The chop gene contains an element for the positive regulation of the mitochondrial unfolded protein response. PLoS One 2:e835
Ryan MT, Hoogenraad NJ (2007) Mitochondrial-nuclear communications. Annu Rev Biochem 76:701–722
Haynes CM, Yang Y, Blais SP, Neubert TA et al (2010) The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell 37:529–540
Haynes CM, Petrova K, Benedetti C, Yang Y et al (2007) ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell 13:467–480
Koll H, Guiard B, Rassow J, Ostermann J et al (1992) Antifolding activity of hsp60 couples protein import into the mitochondrial matrix with export to the intermembrane space. Cell 68:1163–1175
Lee ES, Yoon CH, Kim YS, Bae YS (2007) The double-strand RNA-dependent protein kinase PKR plays a significant role in a sustained ER stress-induced apoptosis. FEBS Lett 581:4325–4332
Nakamura T, Furuhashi M, Li P, Cao H et al (2010) Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 140:338–348
Takada Y, Ichikawa H, Pataer A, Swisher S et al (2007) Genetic deletion of PKR abrogates TNF-induced activation of IkappaBalpha kinase, JNK, Akt and cell proliferation but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene 26:1201–1212
Bence NF, Sampat RM, Kopito RR (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292:1552–1555
Colley NJ, Cassill JA, Baker EK, Zuker CS (1995) Defective intracellular transport is the molecular basis of rhodopsin-dependent dominant retinal degeneration. Proc Natl Acad Sci USA 92:3070–3074
Feldman DE, Chauhan V, Koong AC (2005) The unfolded protein response: a novel component of the hypoxic stress response in tumors. Mol Cancer Res 3:597–605
Koumenis C, Naczki C, Koritzinsky M, Rastani S et al (2002) Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol 22:7405–7416
Blais JD, Filipenko V, Bi M, Harding HP et al (2004) Activating transcription factor 4 is translationally regulated by hypoxic stress. Mol Cell Biol 24:7469–7482
Romero-Ramirez L, Cao H, Nelson D, Hammond E et al (2004) XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res 64:5943–5947
Carrasco DR, Sukhdeo K, Protopopova M, Sinha R et al (2007) The differentiation and stress response factor XBP-1 drives multiple myeloma pathogenesis. Cancer Cell 11:349–360
Erbay E, Babaev VR, Mayers JR, Makowski L et al (2009) Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med 15:1383–1391
Huang CJ, Haataja L, Gurlo T, Butler AE et al (2007) Induction of endoplasmic reticulum stress-induced beta-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab 293:E1656–E1662
Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z et al (2004) Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology 145:5087–5096
Borradaile NM, Han X, Harp JD, Gale SE et al (2006) Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res 47:2726–2737
Shoelson SE, Herrero L, Naaz A (2007) Obesity, inflammation, and insulin resistance. Gastroenterology 132:2169–2180
Harding HP, Zeng H, Zhang Y, Jungries R et al (2001) Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell 7:1153–1163
Delepine M, Nicolino M, Barrett T, Golamaully M et al (2000) EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 25:406–409
Park SW, Zhou Y, Lee J, Lu A et al (2010) The regulatory subunits of PI3 K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med 16:429–437
Winnay JN, Boucher J, Mori MA, Ueki K et al (2010) A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med 16:438–445
Zhou Y, Lee J, Reno CM, Sun C et al (2011) Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat Med 17:356–365
Ozawa K, Miyazaki M, Matsuhisa M, Takano K et al (2005) The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54:657–663
Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K et al (2005) Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 280:847–851
Kammoun HL, Chabanon H, Hainault I, Luquet S et al (2009) GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest 119:1201–1215
Rodriguez A, Duran A, Selloum M, Champy MF et al (2006) Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab 3:211–222
Ebato C, Uchida T, Arakawa M, Komatsu M et al (2008) Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metab 8:325–332
Jung HS, Chung KW, Won Kim J, Kim J et al (2008) Loss of autophagy diminishes pancreatic beta cell mass and function with resultant hyperglycemia. Cell Metab 8:318–324
Billing O, Kao G, Naredi P (2011) Mitochondrial Function Is Required for Secretion of DAF-28/Insulin in C. elegans. PLoS One 6:e14507
Clavel T, Haller D (2007) Bacteria- and host-derived mechanisms to control intestinal epithelial cell homeostasis: implications for chronic inflammation. Inflamm Bowel Dis 13:1153–1164
Shkoda A, Ruiz PA, Daniel H, Kim SC et al (2007) Interleukin-10 blocked endoplasmic reticulum stress in intestinal epithelial cells: impact on chronic inflammation. Gastroenterology 132:190–207
Hampe J, Franke A, Rosenstiel P, Till A et al (2007) A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet 39:207–211
Rioux JD, Xavier RJ, Taylor KD, Silverberg MS et al (2007) Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 39:596–604
(2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature, 447:661–678
Parkes M, Barrett JC, Prescott NJ, Tremelling M et al (2007) Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet 39:830–832
Hugot JP, Chamaillard M, Zouali H, Lesage S et al (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411:599–603
Ogura Y, Bonen DK, Inohara N, Nicolae DL et al (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411:603–606
Franchimont D, Vermeire S, El Housni H, Pierik M et al (2004) Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn’s disease and ulcerative colitis. Gut 53:987–992
Cadwell K, Liu JY, Brown SL, Miyoshi H et al (2008) A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal paneth cells. Nature 456:259–263
Wehkamp J, Salzman NH, Porter E, Nuding S et al (2005) Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc Natl Acad Sci USA 102:18129–18134
Kaser A, Blumberg RS (2008) Paneth cells and inflammation dance together in Crohn’s disease. Cell Res 18:1160–1162
Werner T, Wagner SJ, Martinez I, Walter J et al (2011) Depletion of luminal iron alters the gut microbiota and prevents Crohn’s disease-like ileitis. Gut 60:325–333
Vecchi C, Montosi G, Zhang K, Lamberti I et al (2009) ER stress controls iron metabolism through induction of hepcidin. Science 325:877–880
Beltran B, Nos P, Dasi F, Iborra M et al (2010) Mitochondrial dysfunction, persistent oxidative damage, and catalase inhibition in immune cells of naive and treated Crohn’s disease. Inflamm Bowel Dis 16:76–86
He D, Sougioultzis S, Hagen S, Liu J et al (2002) Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 122:1048–1057
Kamizato M, Nishida K, Masuda K, Takeo K et al (2009) Interleukin 10 inhibits interferon gamma- and tumor necrosis factor alpha-stimulated activation of NADPH oxidase 1 in human colonic epithelial cells and the mouse colon. J Gastroenterol 44:1172–1184
Lewis K, Lutgendorff F, Phan V, Soderholm JD et al (2010) Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis 16:1138–1148
Nazli A, Yang PC, Jury J, Howe K et al (2004) Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol 164:947–957
Soderholm JD, Olaison G, Peterson KH, Franzen LE et al (2002) Augmented increase in tight junction permeability by luminal stimuli in the non-inflamed ileum of Crohn’s disease. Gut 50:307–313
Schurmann G, Bruwer M, Klotz A, Schmid KW et al (1999) Transepithelial transport processes at the intestinal mucosa in inflammatory bowel disease. Int J Colorectal Dis 14:41–46
Silva AM, Wang D, Komar AA, Castilho BA et al (2007) Salicylates trigger protein synthesis inhibition in a protein kinase R-like endoplasmic reticulum kinase-dependent manner. J Biol Chem 282:10164–10171
Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM et al (2001) Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor gamma and mediated by inhibition of translation initiation. Cancer Res 61:6213–6218
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M et al (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313:1137–1140
Ota T, Gayet C, Ginsberg HN (2008) Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest 118:316–332
Boyce M, Bryant KF, Jousse C, Long K et al (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307:935–939
Sokka AL, Putkonen N, Mudo G, Pryazhnikov E et al (2007) Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci 27:901–908
Palacios HH, Yendluri BB, Parvathaneni K, Shadlinski VB et al (2011) Mitochondrion-specific antioxidants as drug treatments for Alzheimer disease. CNS Neurol Disord Drug Targets 10:149–162
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rath, E., Haller, D. Inflammation and cellular stress: a mechanistic link between immune-mediated and metabolically driven pathologies. Eur J Nutr 50, 219–233 (2011). https://doi.org/10.1007/s00394-011-0197-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0197-0