Abstract
Background
Previous human studies on the effect of dietary calcium supplementation on faecal excretion of bile acids (BA) and faecal water concentrations of animal neutral sterols (NSt, cholesterol and its metabolites) lack detailed information about single BA and NSt.
Aim of the study
We investigated whether single BA and NSt in faeces and especially in faecal water are affected by calcium supplementation and whether this affects genotoxicity of faecal water. In addition, we differentiated between men and women with regard to the concentrations of BA and NSt in faecal water.
Methods
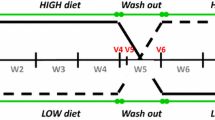
Thirty-one healthy volunteers consumed a calcium supplemented bread (1.0 g/day) and a placebo bread, respectively, for 4 weeks in a double-blind, randomised cross-over trial. Faeces were collected quantitatively for 5 days in the last week of each period. NSt and BA were analysed by GC–MS.
Results
Due to calcium supplementation faecal concentrations of lithocholic acid (LCA, 14%, P = 0.008), deoxycholic acid (DCA, 19%, P < 0.001) and 12keto-deoxycholic acid (12keto DCA, 29%, P = 0.049) significantly increased whereas BA concentrations in faecal water were only marginally affected. In contrast, concentrations of cholesterol (30%, P = 0.020) and its metabolites coprostanol (43%, P = 0.004), coprostanone (36%, P = 0.003), cholestanol (44%, P = 0.001) and cholestenone (32%, P = 0.038) in faecal water significantly decreased. Total NSt concentration in faecal water was found to be significantly higher in women compared to men (P = 0.018). The genotoxicity of faecal water was neither affected by calcium supplementation nor were there gender-specific differences.
Conclusions
Dietary calcium supplementation diversely affects BA and NSt in faeces and in faecal water but does not influence the genotoxicity of faecal water in healthy adults.
Similar content being viewed by others
References
Block JB, Dietrich MF, Jawaid S, Gilgomez K, Morrison L, Rucker P (1987) Inhibition of fecapentaene-12 and carcinogen induced mutagenesis in the Ames test by carageenans and calcium. Proc Am Assoc Cancer Res 28:103
Burger HG (2002) Androgen production in women. Fertil Steril 77(Suppl 4):S3–S5
Chiang JYL (2003) Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastroint Liver Physiol 284:349–356
De Kok TMCM, van Faassen A, Glinghammer B, Pachen DMFA, Eng M, Rafter JJ, Baeten CGMI, Engels LGJB, Kleinjans JCS (1999) Bile acid concentrations, cytotoxicity, and pH of faecal water from patients with colorectal adenomas. Dig Dis Sci 44:2218–2225
De Kok TMCM, Vaniersel MLPS, Tenhoor F, Kleinjans JCS (1993) In vitro study on the effects of faecal composition on fecapentaene kinetics in the large bowel. Mutation Res 302:103–108
Ditscheid B, Keller S, Jahreis G (2005) Cholesterol metabolism is affected by calcium phosphate supplementation in humans. J Nutr 135:1678–1682
Dorgan JF, Fears TR, McMahon RP, Aronson Friedman L, Patterson BH, Greenhut SF (2002) Measurement of steroid sex hormones in serum: a comparison of radioimmunoassay and mass spectrometry. Steroids 67:151–158
dos Santos Silva I, Swerdlow AJ (1993) Sex differences in the risks of hormone-dependent cancers. Am J Epidemiol 138:10–28
Geltner Allinger U, Johansson GK, Gustafsson JÅ, Rafter J (1989) Shift from a mixed to a lactovegetarian diet: influence on acidic lipids in faecal water—a potential risk factor for colon cancer. Am J Clin Nutr 50:992–996
Glinghammar B, Inoue H, Rafter JJ (2002) Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-kB and AP-1. Carcinogenesis 23:839–845
Glinghammar B, Venturi M, Rowland IR, Rafter JJ (1997) Shift from a dairy product-rich to a dairy product-free diet: influence on cytotoxicity and genotoxicity of faecal water–potential risk markers for colon cancer. Am J Clin Nutr 66:1277–1282
Govers MJAP, Termont DSML, Lapré JA, Kleibeuker JH, Vonk RJ, van der Meer R (1996) Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic cytotoxicity in humans. Cancer Res 56:3270–3275
Govers MJAP, Termont DSML, van Aken GA, van der Meer R (1994) Characterization of the adsorption of conjugated and unconjugated bile acids to insoluble, amorphous calcium phosphate. J Lipid Res 35:741–748
Hayashi E, Amuro Y, Endo T, Yamamoto H, Miyamoto M, Kishimoto S (1986) Fecal bile acids and neutral sterols in rats with spontaneous colon cancer. Int J Cancer 37:629–632
Hofmann AF (1999) The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med 159:2647–2658
Hylla S, Gostner A, Dusel G, Anger H, Bartram HP, Christl SU, Kasper H, Scheppach W (1998) Effects of resistant starch on the colon in healthy volunteers: possible implications for cancer prevention. Am J Clin Nutr 67:136–142
Keller S, Jahreis G (2004) Determination of underivatised sterols and bile acid trimethyl silyl ether methyl esters by gas chromatography-mass spectrometry-single ion monitoring in faeces. J Chromatogr B 813:199–207
Korpela JT, Adlercreutz H, Turunen MJ (1988) Fecal free and conjugated bile acids and neutral sterols in vegetarians, omnivores, and patients with colorectal cancer. Scand J Gastroenterol 23:277–283
Lampe JW, Fredstrom SB, Slavin JL, Potter JD (1993) Sex-differences in colonic function—a randomized trial. Gut 34:531–536
Lapré JA, deVries HT, Termont DSML, Kleibeuker JH, deVries EGE, van der Meer R (1993) Mechanism of the protective effect of supplemental dietary calcium on cytolytic activity of faecal water. Cancer Res 53:248–253
Lupton JR, Steinbach G, Chang WC, O’Brien BC, Wiese S, Stoltzfus CL, Glober GA, Wargovich MJ, McPherson RS, Winn RJ (1996) Calcium supplementation modifies the relative amounts of bile acids in bile and affects key aspects of human colon physiology. J Nutr 126:1421–1428
McMichael AJ, Potter JD (1983) Do intrinsic sex differences in lower alimentary tract physiology influence the sex-specific risks of bowel cancer and other biliary and intestinal diseases? Am J Epidemiol 118:620–627
Midtvedt T, Lingaas E, Carlstedt-Duke B, Höverstad T, Midtvedt AC, Saxerholt H, Steinbakk M, Norin KE (1990) Intestinal microbial conversion of cholesterol to coprostanol in man. APMIS 98:839–844
Nagengast FM, Grubben MJAL, van Munster IP (1995) Role of bile acids in colorectal carcinogenesis. Eur J Cancer 31A:1067–1070
Nair PP (1988) Role of bile acids and neutral sterols in carcinogenesis. Am J Clin Nutr 48:768–774
Nordling MM, Glinghammar B, Karlsson PC, de Kok TMCM, Rafter JJ (2003) Effects of cell proliferation, activator protein-1 and genotoxicity by faecal water from patients with colorectal adenomas. Scand J Gastroenterol 38:549–555
Osswald K, Becker TW, Grimm M, Jahreis G, Pool-Zobel BL (2000) Inter- and intra-individual variation of faecal water–genotoxicity in human colon cells. Mutation Res 472:59–70
Powolny A, Xu J, Loo G (2001) Deoxycholate induces DNA damage and apoptosis in human colon epithelial cells expressing either mutant or wild-type p53. Int J Biochem Cell Biol 33:193–203
Price JF, Lee AJ, Fowkes FGR (1997) Steroid sex hormones and peripheral arterial disease in the Edinburgh Artery Study. Steroids 62:789–794
Rafter JJ, Child P, Anderson AM, Alder R, Eng V, Bruce WR (1987) Cellular toxicity of faecal water depends on diet. Am J Clin Nutr 45:559–563
Ren D, Li L, Schwabacher AW, Young JW, Beitz DC (1996) Mechanism of cholesterol reduction to coprostanol by Eubacterium coprostanoligenes ATCC 51222. Steroids 61:33–34
Rieger MA, Parlesak A, Pool-Zobel BL, Rechkemmer G, Bode C (1999) A diet high in fat and meat but low in dietary fibre increases the genotoxic potential of ‘faecal water’. Carcinogenesis 20:2311–2316
Roberton AM (1993) Roles of endogenous substances and bacteria in colorectal cancer. Mutation Res 290:71–78
Roy P, Owen RW, Faivre J, Scheppach W, Saldanha MH, Beckly DE, Boutron MC (1999) Faecal neutral sterols and bile acids in patients with adenomas and large bowel cancer: an EPC case-control study. European cancer prevention. Eur J Cancer Prev 8:409–415
Stadler J, Stern HS, Yeung KS, McGuire V, Furrer R, Marcon N, Bruce WR (1988) Effect of high fat consumption on cell proliferation activity of colorectal mucosa and on soluble faecal bile acids. Gut 29:1326–1331
Stadler J, Yeung KS, Furrer R, Marcon N, Himal HS, Bruce WR (1988) Proliferative activity of rectal mucosa and soluble faecal bile acids in patients with normal colons and in patients with colonic polyps or cancer. Cancer Lett 38:315–320
Suzuki K, Bruce RW, Baptista J, Furrer R, Vaughan DJ, Krepinsky JJ (1986) Characterization of cytotoxic steroids in human faeces and their putative role in the etiology of human colonic cancer. Cancer Lett 33:307–316
Ten Bruggencate SJM, Bovee-Oudenhoven IMJ, Lettink-Wissink MLG, Katan MB, Van der Meer R (2004) Dietary fructo-oligosaccharides and inulin decrease resistance of rats to salmonella: protective role of calcium. Gut 53:530–535
Termine JD, Posner AS (1970) Calcium phosphate formation in vitro. I. Factors affecting initial phase separation. Arch Biochem Biophys 140:307–317
Van der Meer R, de Vries HT (1985) Differential binding of glycine- and taurine-conjugated bile acids to insoluble calcium phosphate. Biochem J 229:265–268
Van der Meer R, Lapré JA, Govers MJAP, Kleibeuker JH (1997) Mechanisms of the intestinal effects of dietary fats and milk products on colon carcinogenesis. Cancer Lett 114:75–83
Van der Meer R, Welberg JWM, Kuipers F, Kleibeuker JH, Mulder NH, Termont DSML, Vonk RJ, de Vries HT, de Vries EGE (1990) Effect of supplemental dietary calcium on the intestinal association of calcium, phosphate and bile acids. Gastroenterol 99:1653–1659
Van Faassen A, Hazen MJ, van den Brandt PA, van den Bogaard AE, Hermus RJJ, Janknegt RA (1993) Bile acids and pH values in total faeces and in faecal water from habitually omnivorous and vegetarian subjects. Am J Clin Nutr 58:917–922
Van Gorkom BAP, van der Meer R, Boersma-van Ek W, Termont DSML, de Vries EGE, Kleibeuker JH (2002) Changes in bile acid composition and effect on cytolytic activity of faecal water by ursodeoxycholic acid administration: a placebo-controlled cross-over intervention trial in healthy volunteers. Scand J Gastroenterol 37:965–971
Van Munster IP, Tangerman A, Nagengast FM (1994) Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Dig Dis Sci 39:834–842
Venturi M, Hambly RJ, Glinghammar B, Rafter JJ, Rowland IR (1997) Genotoxic activity in human faecal water and the role of bile acids: a study using the alkaline comet assay. Carcinogenesis 18:2353–2359
Weisburger JH, Reddy BS, Barnes WS, Wynder EL (1983) Bile acids, but not neutral sterols, are tumor promoters in the colon in man and in rodents. Environ Health Persp 50:101–107
Weststrate JA, Ayesh R, Bauer-Plank C, Drewitt PN (1999) Safety evaluation of phytosterol esters. Part 4. Faecal concentrations of bile acids and neutral sterols in healthy normolipidaemic volunteers consuming a controlled diet either with or without a phytosterol ester-enriched margarine. Food Chem Toxicol 37:1063–1071
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ditscheid, B., Keller, S. & Jahreis, G. Faecal steroid excretion in humans is affected by calcium supplementation and shows gender-specific differences. Eur J Nutr 48, 22–30 (2009). https://doi.org/10.1007/s00394-008-0755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-008-0755-2