Abstract
Background
Conflicting evidence suggests a possible role for vitamin E in mammalian glucose metabolism and the protection from type 2 diabetes. The alpha-tocopherol transfer protein (α-TTP) mediates the transfer of α-tocopherol (α-TOH) from hepatocytes to very-low-density lipoproteins, thereby controlling plasma levels of α-TOH.
Aim of the study
The aim of this study was to investigate the putative impact of α-TTP knock-out on glucose metabolism in mice.
Methods
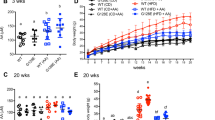
Mice deficient for α-TTP and wild-type control littermates were fed a diet containing 200 mg α-tocopheryl acetate per kg to ameliorate α-TOH deficiency in knock-out mice. We investigated fasting and postprandial plasma glucose, insulin and triglyceride levels of both groups of mice at different ages. All genotypes and age groups were further subjected to glucose and insulin tolerance tests, and number of insulin-producing islets of Langerhans were determined.
Results
Plasma α-TOH levels of knock-out mice were 34% the levels of wild-type controls: Any signs of α-TOH deficiency were absent at any age. Unexpectedly, serum glucose levels both in the fasted and in the fed state were lower in α-TTP-deficient mice at any age. Removal rates for intraperitoneally injected glucose were found to be significantly increased in young α-TTP-deficient mice. This improved glucose tolerance was caused by increased insulin secretion in response to an intraperitoneal glucose challenge due to an increased number of pancreatic islets, as well as by increased sensitivity to intraperitoneally injected insulin, both significantly promoting glucose metabolism in α-TTP-deficient mice.
Conclusions
Our findings suggest that α-TTP-deficiency in states of α-TOH supplementation unexpectedly promotes glucose tolerance in mice due to both increased insulin secretion and insulin action, suggesting differential roles of α-TTP and α-TOH in the pathogenesis of type 2 diabetes mellitus.




Similar content being viewed by others
References
Arita M, Sato Y, Miyata A, Tanabe T, Takahashi E, Kayden HJ, Arai H, Inoue K (1995) Human alpha-tocopherol transfer protein: cDNA cloning, expression and chromosomal localization. Biochem J 306:437–443
Azzi A, Ricciarelli R, Zingg JM (2002) Non-antioxidant molecular functions of alpha-tocopherol (vitamin E). FEBS Lett 519:8–10
Brigelius-Flohe R (2006) Bioactivity of vitamin E. Nutr Res Rev 19:174–186
Brigelius-Flohe R, Kelly FJ, Salonen JT, Neuzil J, Zingg JM, Azzi A (2002) The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr 76:703–716
Chidakel A, Mentuccia D, Celi FS (2005) Peripheral metabolism of thyroid hormone and glucose homeostasis. Thyroid 15:899–903
Copp RP, Wisniewski T, Hentati F, Larnaout A, Ben Hamida M, Kayden HJ (1999) Localization of alpha-tocopherol transfer protein in the brains of patients with ataxia with vitamin E deficiency and other oxidative stress related neurodegenerative disorders. Brain Res 822:80–87
Czernichow S, Couthouis A, Bertrais S, Vergnaud AC, Dauchet L, Galan P, Hercberg S (2006) Antioxidant supplementation does not affect fasting plasma glucose in the supplementation with antioxidant vitamins and minerals (SU.VI.MAX) study in France: association with dietary intake and plasma concentrations. Am J Clin Nutr 84:395–399
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Dufresne AM, Smith RJ (2005) The adapter protein GRB10 is an endogenous negative regulator of insulin-like growth factor signaling. Endocrinology 146:4399–4409
Evans HM, Bishop KS (1922) On the existence of a hithero unrecognized dietary factor essential for reproduction. Science 56:650–651
Feskens EJ, Virtanen SM, Rasanen L, Tuomilehto J, Stengard J, Pekkanen J, Nissinen A, Kromhout D (1995) Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the seven countries study. Diabetes Care 18:1104–1112
Frei B (1994) Reactive oxygen species and antioxidant vitamins: mechanisms of action. Am J Med 97:5S–13S, Discussion 22S–28S
Gohil K, Schock BC, Chakraborty AA, Terasawa Y, Raber J, Farese RV Jr, Packer L, Cross CE, Traber MG (2003) Gene expression profile of oxidant stress and neurodegeneration in transgenic mice deficient in alpha-tocopherol transfer protein. Free Radic Biol Med 35:1343–1354
Haluzik MM, Haluzik M (2006) PPAR-alpha and insulin sensitivity. Physiol Res 55:115–122
Hayton SM, Kriss T, Wade A, Muller DP (2006) Effects on neural function of repleting vitamin E-deficient rats with alpha-tocopherol. J Neurophysiol 95:2553–2559
Heald AH, Kaushal K, Siddals KW, Rudenski AS, Anderson SG, Gibson JM (2006) Insulin-like growth factor binding protein-2 (IGFBP-2) is a marker for the metabolic syndrome. Exp Clin Endocrinol Diabetes 114:371–376
Isken F, Schulz TJ, Möhlig M, Pfeiffer AF, Ristow M (2006) Chemical inhibition of citrate metabolism alters glucose metabolism in mice. Horm Metab Res 38:543–545
Isken F, Schulz TJ, Weickert MO, Pfeiffer AF, Ristow M (2006) Chemical inhibition of citrate metabolism alters body fat content in mice. Horm Metab Res 38:134–136
Jishage K, Arita M, Igarashi K, Iwata T, Watanabe M, Ogawa M, Ueda O, Kamada N, Inoue K, Arai H, Suzuki H (2001) alpha-tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. J Biol Chem 276:1669–1672
Kaempf-Rotzoll DE, Igarashi K, Aoki J, Jishage K, Suzuki H, Tamai H, Linderkamp O, Arai H (2002) Alpha-tocopherol transfer protein is specifically localized at the implantation site of pregnant mouse uterus. Biol Reprod 67:599–604
Kluth D, Landes N, Pfluger P, Muller-Schmehl K, Weiss K, Bumke-Vogt C, Ristow M, Brigelius-Flohe R (2005) Modulation of Cyp3a11 mRNA expression by alpha-tocopherol but not gamma-tocotrienol in mice. Free Radic Biol Med 38:507–514
Knekt P, Reunanen A, Marniemi J, Leino A, Aromaa A (1999) Low vitamin E status is a potential risk factor for insulin-dependent diabetes mellitus. J Intern Med 245:99–102
Koya D, Lee IK, Ishii H, Kanoh H, King GL (1997) Prevention of glomerular dysfunction in diabetic rats by treatment with d-alpha-tocopherol. J Am Soc Nephrol 8:426–435
Kunisaki M, Bursell SE, Clermont AC, Ishii H, Ballas LM, Jirousek MR, Umeda F, Nawata H, King GL (1995) Vitamin E prevents diabetes-induced abnormal retinal blood flow via the diacylglycerol-protein kinase C pathway. Am J Physiol 269:E239–E246
Lavis VR, Kitabchi AE, Williams RH (1969) Lipid peroxidation in vitro by isolated fat cells of rats. Correlation with total lipolysis, glucose utilization, and dietary tocopherol. J Biol Chem 244:4382–4386
Leonard SW, Terasawa Y, Farese RV Jr, Traber MG (2002) Incorporation of deuterated RRR- or all-rac-alpha-tocopherol in plasma and tissues of alpha-tocopherol transfer protein—null mice. Am J Clin Nutr 75:555–560
Lonn E, Yusuf S, Hoogwerf B, Pogue J, Yi Q, Zinman B, Bosch J, Dagenais G, Mann JF, Gerstein HC (2002) Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care 25:1919–1927
McKusick VA (2006) Familial isolated deficiency of vitamin E, http://www.ncbi.nlm.nih.gov/htbin-post/Omim/dispmim?277460
Montonen J, Knekt P, Jarvinen R, Reunanen A (2004) Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 27:362–366
Muller-Schmehl K, Beninde J, Finckh B, Florian S, Dudenhausen JW, Brigelius-Flohe R, Schuelke M (2004) Localization of alpha-tocopherol transfer protein in trophoblast, fetal capillaries’ endothelium and amnion epithelium of human term placenta. Free Radic Res 38:413–420
Ouahchi K, Arita M, Kayden H, Hentati F, Ben Hamida M, Sokol R, Arai H, Inoue K, Mandel JL, Koenig M (1995) Ataxia with isolated vitamin E deficiency is caused by mutations in the alpha-tocopherol transfer protein. Nat Genet 9:141–145
Pomplun D, Florian S, Schulz T, Pfeiffer AF, Ristow M (2007) Alterations of pancreatic beta-cell mass and islet number due to Ins2-controlled expression of cre recombinase: RIP-cre revisited, part 2. Horm Metab Res 39:336–340
Ristow M (2004) Neurodegenerative disorders associated with diabetes mellitus. J Mol Med 82:510–529
Ristow M, Mulder H, Pomplun D, Schulz TJ, Müller-Schmehl K, Krause A, Fex M, Puccio H, Müller J, Isken F, Spranger J, Müller-Wieland D, Magnuson MA, Möhlig M, Koenig M, Pfeiffer AFH (2003) Frataxin-deficiency in pancreatic islets causes diabetes due to loss of beta-cell mass. J Clin Invest 112:527–534
Sacco M, Pellegrini F, Roncaglioni MC, Avanzini F, Tognoni G, Nicolucci A (2003) Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care 26:3264–3272
Salonen JT, Nyyssonen K, Tuomainen TP, Maenpaa PH, Korpela H, Kaplan GA, Lynch J, Helmrich SP, Salonen R (1995) Increased risk of non-insulin dependent diabetes mellitus at low plasma vitamin E concentrations: a four year follow up study in men. Bmj 311:1124–1127
Sulzle A, Hirche F, Eder K (2004) Thermally oxidized dietary fat upregulates the expression of target genes of PPAR alpha in rat liver. J Nutr 134:1375–1383
Tasinato A, Boscoboinik D, Bartoli GM, Maroni P, Azzi A (1995) d-alpha-tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proc Natl Acad Sci USA 92:12190–12194
Terasawa Y, Ladha Z, Leonard SW, Morrow JD, Newland D, Sanan D, Packer L, Traber MG, Farese RV (2000) Increased atherosclerosis in hyperlipidemic mice deficient in alpha-tocopherol transfer protein and vitamin E. Proc Natl Acad Sci USA 97:13830–13834
Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T (2005) Peroxisome proliferator-activated receptor (PPAR) alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes 54:3358–3370
Yokota T, Igarashi K, Uchihara T, Jishage K, Tomita H, Inaba A, Li Y, Arita M, Suzuki H, Mizusawa H, Arai H (2001) Delayed-onset ataxia in mice lacking alpha-tocopherol transfer protein: model for neuronal degeneration caused by chronic oxidative stress. Proc Natl Acad Sci USA 98:15185–15190
Zimmer S, Stocker A, Sarbolouki MN, Spycher SE, Sassoon J, Azzi A (2000) A novel human tocopherol-associated protein: cloning, in vitro expression, and characterization. J Biol Chem 275:25672–25680
Acknowledgments
The authors wish to thank Dr. Robert V. Farese for generously providing α-TTP knock-out mice. The excellent technical assistance of Susann Richter and Elke Thom is gratefully acknowledged. This study was supported by a grant of the Deutsche Forschungsgemeinschaft and the Wilhelm-Sander-Stiftung (both to M.R.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Marc Birringer and Doreen Kuhlow have contributed equally to this publication.
Rights and permissions
About this article
Cite this article
Birringer, M., Kuhlow, D., Pfluger, P.T. et al. Improved glucose metabolism in mice lacking α-tocopherol transfer protein. Eur J Nutr 46, 397–405 (2007). https://doi.org/10.1007/s00394-007-0679-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-007-0679-2