Summary
Background
Neural tube defects (NTD) are mainly of multifactorial origin. Maternal treatment with valproic acid (VPA) during pregnancy induces NTD in susceptible fetuses. Elevated levels of homocysteine are observed in pregnancies with NTD. The mechanism by which homocysteine might cause NTD is unknown.
Aim of the Study
The aim of this study was to determine if homocystine would augment VPA-induced exencephaly in an experimental model.
Methods
Groups of mice were injected (IP) on gestational day 8 (GD) with a single dose of 75 mg/kg of l-Homocystine (HC) or a proportionate volume of saline, followed by a single dose of 600 mg/kg of VPA or an equal volume of saline. In a second experiment, mice were treated with a daily dose of 75 mg/kg of HC or an equal volume of saline (IP) from GD 5 and continued through GD 10. These animals had a single exposure to 600 mg/kg of VPA or saline (IP) on GD 8. All animals were killed by cervical dislocation on GD 18. Plasma homocysteine, folate and vitamin B12 were determined on GD 8 and GD 10 from single and multiple dose groups of mice, respectively, from additional experiments.
Results
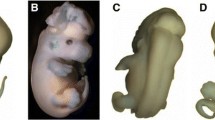
The VPA and HC+VPA induced significantly higher rates of embryonic resorption and intrauterine growth retardation (IUGR) than HC or saline alone. HC + VPA groups had significantly more numerous fetuses with severe IUGR than HC alone or VPA alone groups. Both single and multiple doses of HC augmented VPA-induced reduction in fetal body weight. Successive doses of HC did not augment the rate of IUGR more significantly than a single dose of HC. Incidence of exencephaly was significantly enhanced in the HC + VPA groups compared to that in the HC or VPA alone groups. HC alone was not teratogenic. Plasma homocysteine levels increased several fold both in HC and HC + VPA groups and the increase was not particularly more marked in multiple dose groups than in the single dose groups. VPA did not elevate homocysteine concentration. Both FA and vitamin B12 concentrations were reduced by VPA, HC and HC + VPA, but HC and VPA when combined did not produce an additive effect on vitamin levels.
Conclusion
These data indicate that HC and VPA interact in neurulation stage embryos, affect fundamental processes of closure of the neural tube and lead to enhanced incidence of NTD.

Similar content being viewed by others
References
Botto LD, Moore CA, Khoury MJ, Erickson JD (1999) Neural-tube defects. N Engl J Med 341:1509–15019
Cherian A, Seena S Bullock RK, Antony PC (2005) Incidence of neural tube defects in the least-developed area of India: a population-based study. Lancet 366(9489):930–931
Sulik KK, Sadler TW (1993) Postulated mechanisms underlying the development of neural tube defects. Insights from in vitro and in vivo studies. Ann N Y Acad Sci 15(678):8–21
Frey L, Hauser WA (2003) Epidemiology of neural tube defects. Epilepsia 44(Suppl 3):4–13
Holmes LB (1994) Spina bifida: anticonvulsants and other maternal influences. Ciba Found Symp 181:232–238
Robert E (1988) Valproic acid as a human teratogen. Cong Anom 28(Suppl):S71–S80
Lindhout D, Meinardi H, Meijer JWA, Nau H (1992) Antiepileptic drugs and teratogenesis in two consecutive cohorts: Changes in prescription policy, paralleled by changes in pattern of malformations. Neurology 42(suppl): 94–110
Padmanabhan R, Ahmad I (1996) Sodium valproate augments spontaneous neural tube defects and axial skeletal malformations in TO mouse fetuses. Reproduct Toxicol 10:345–363
Ardinger HH, Atkin JF, Blackston RD, Elsas LJ, Clarren SK, Livingston S, Flanery DB, Pellock JM, Harrod MJ, Lammer EJ, Majewsky F, Schinzel A, Roriello HV, Hansen JW (1988) Verification of the fetal valproate syndrome phenotype. Am J Med Genet 29:171–185
Smithells RW, Sheppard S, Schorah CJ, Seller MJ, Nevin NC, Harris R, Read AP, Fielding DW (1981) Apparent prevention of neural tube defects by periconceptional vitamin supplementation. Arch Dis Child 56:911–918
MRC Vitamin Study Research Group (1991) Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 338(8760):131–137
Czeizel AE, Dudas I (1992) Prevention of the first occurrence of neural tube defects by periconceptional vitamin supplementation. New Eng J Med 327:1832–1835
McMullin MF, Young PB, Bailie KE, Savage GA, Lappin TR, White R (2001) Homocysteine and methylmalonic acid as indicators of folate and vitamin B12 deficiency in pregnancy. Clin Lab Haematol 23:161–165
Biale Y, Lewenthal H (1984) Effect of folic acid supplementation on congenital malformations due to anticonvulsive drugs. Eur J Obstet Gynecol Reprod Biol 18:211–216
Champel V, Radal M, Moulin-Vallez M, Jonville-Bera AP, Autret-Leca E, Champel V, Radal M, Moulin-Vallez M, Jonville-Bera AP, Autret-Leca E (1999) Should folic acid be given to women treated with valproic acid and/or carbamazepine? Folic acid and pregnancy in epilepsy. Rev Neurol (Paris) 155:220–224
Heil SG, van der Put NM, Trijbels FJ, Gabreels FJ, Blom HJ (1999) Molecular genetic analysis of human folate receptors in neural tube defects. Eur J Hum Genet 7:393–396
Steegers-Theunissen RP, Boers GH, Blom HJ, Nijhuis JG, Thomas CM, Borm GF, Eskes TK (1995) Neural tube defects and elevated homocysteine levels in amniotic fluid. Am J Obstet Gynecol 172:1436–1441
Wenstrom KD, Johanning GL, Owen J, Johnston KE, Acton S, Tamura T (2000) Role of amniotic fluid homocysteine level and of fetal 5, 10-methylenetetrahydrafolate reductase genotype in the etiology of neural tube defects. Am J Med Genet 90(1):12–16
Apeland T, Mansoor MA, Strandjord RE (2001) Antiepileptic drugs as independent predictors of plasma total homocysteine levels. Epilepsy Res 47(1–2):27–35
Afman LA, Blom HJ, Van der Put NM, Van Straaten HW (2003) Homocysteine interference in neurulation: a chick embryo model. Birth Defects Res Part A Clin Mol Teratol 67:421–428
Epeldegui M, Pena-Melian A, Varela-Moreiras G, Perez-Miguelsanz J (2002) Homocysteine modifies development of neurulation and dorsal root ganglia in chick embryos. Teratology 65:171–179
Hansen DK, Grafton TF, Melnyk S, James SJ (2001) Lack of embryotoxicity of homocysteine thiolactone in mouse embryos in vitro. Reprod Toxicol 15:239–244
Greene ND, Dunlevy LE, Copp AJ (2003) Homocysteine is embryotoxic but does not cause neural tube defects in mouse embryos. Anat Embryol (Berl) 206:185–191
Padmanabhan R, Shafiullah MM (2003) Amelioration of sodium valproate-induced neural tube defects in mouse fetuses by maternal folic acid supplementation during gestation. Cong Anom 43:29–40
Padmanabhan R, Ibrahim A, Bener A (2002) Effect of maternal methionine pre-treatment on alcohol-induced exencephaly and axial skeletal dysmorphogenesis in mouse fetuses. Drug Alcohol Depend 65(3):263–281
Commission on Life Sciences (1996) Guide for the care and use of laboratory animals. Institute of Laboratory Animal Resources National Research Council National Academy Press, Washington, D.C
Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM (1993) Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med 86(11):703–708
Bower C, Stanley FJ, Croft M, de Klerk N, Davis RE, Nicol DJ (1993) Absorption of pteroylpolyglutamates in mothers of infants with neural tube defects. Br J Nutr 69(3):827–834
Shields DC, Kirke PN, Mills JL, Ramsbottom D, Molloy AM, Burke H, Weir DG, Scott JM, Whitehead AS (1999) The “thermolabile” variant of methylenetetrahydrofolate reductase and neural tube defects: An evaluation of genetic risk and the relative importance of the genotypes of the embryo and the mother. Am J Hum Genet 64:1045–1055
Spiegelstein O, Mitchell LE, Merriweather MY, Wicker NJ, Zhang Q, Lammer EJ, Finnell RH (2004) Embryonic development of folate binding protein-1 (Folbp1) knockout mice: Effects of the chemical form, dose, and timing of maternal folate supplementation. Dev Dyn 231(1):221–231
Yoo JH, Hong SB (1999) A common mutation in the methylenetetrahydrofolate reductase gene is a determinant of hyperhomocysteinemia in epileptic patients receiving anticonvulsants. Metabolism 48(8):1047–1051
Vanaerts LA, Blom HJ, Deabreu RA, Trijbels FJ, Eskes TK, Copius Peereboom-Stegeman JH, Noordhoek J (1994) Prevention of neural tube defects by and toxicity of l-homocysteine in cultured postimplantation rat embryos. Teratology 50:348–360
Snow MH, Tam PP (1979) Is compensatory growth a complicating factor in mouse teratology? Nature 279(5713):555–557
Southern F, Eidt J, Drouilhet J, Mukunyadzi P, Williams DK, Cruz C, Wang YF, Poirier LA, Brown AT, Moursi MM (2001) Increasing levels of dietary homocystine with carotid endarterectomy produced proportionate increases in plasma homocysteine and intimal hyperplasia. Atherosclerosis 158:129–138
Pippenger CE (2003) Pharmacology of neural tube defects. Epilepsia 44(Suppl 3):24–32
Carl GF (1986) Effect of chronic valproate treatment on folate-dependent methyl biosynthesis in the rat. Neurochem Res 11(5):671–85
Ubeda N, Alonso-Aperte E, Varela-Moreiras G (2002) Acute valproate administration impairs methionine metabolism in rats. J Nutr 132(9):2737–2742
Rao ML, Stefan H, Scheid C, Kuttler AD, Froscher W (1993) Serum amino acids, liver status, and antiepileptic drug therapy in epilepsy. Epilepsia 34(2):347–354
Heydrick SJ, Weiss N, Thomas SR, Cap AP, Pimentel DR, Loscalzo J, Keaney JF Jr (2004) l-Homocysteine and l-homocystine stereospecifically induce endothelial nitric oxide synthase-dependent lipid peroxidation in endothelial cells. Free Radic Biol Med 36(5):632–640
Matthews RG, Sheppard C, Goulding C (1998) Methylenetetrahydrofolate reductase and methionine synthase: biochemistry and molecular biology. Eur J Pediatr 157(Suppl 2):S54–S59
De Franchis R, Sperandeo MP, Sebastio G, Andria G (1998) Clinical aspects of cystathionine beta-synthase deficiency: how wide is the spectrum? The Italian Collaborative Study Group on Homocystinuria. Eur J Pediatr 157(Suppl 2):S67–S70
Eskes TKAB (1998) Neural tube defects, vitamins and homocysteine. Eur J Pediatr 157(Suppl 2):S139–S141
Rosenquist TH, Schneider AM, Monogham DT (1999) N-methyl-d-aspartate receptor agonists modulate homocysteine-induced developmental abnormalities. FASEB J 13:1523–1531
Acknowledgments
This work was supported by generous grants (FMHS, NP/2000/25) from the Faculty of Medicine and Health Sciences of the UAE University. The expert technical assistance of Mr. Alsajir Mohammed is appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Padmanabhan, R., Shafiullah, M., Benedict, S. et al. Effect of maternal exposure to homocystine on sodium valproate-induced neural tube defects in the mouse embryos. Eur J Nutr 45, 311–319 (2006). https://doi.org/10.1007/s00394-006-0600-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-006-0600-4