Abstract
Introduction
This study compared the frequency and severity of depressive disorders in patients with antineutrophil cytoplasmic antibody-associated vasculitis (AAV) before and during the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) pandemic using the Korean version of the Center for Epidemiologic Studies Depression Scale-Revised (K-CESD-R) and the Korean version of the Profile of Mood States (K-POMS) depression, and further determined predictors of current depressive disorders in the patients during the pandemic.
Methods
Of the 61 patients with AAV who participated before the pandemic, 8 patients were transferred to other hospitals, 3 patients died, and 2 patients refused to participate in this study. Finally, 48 patients participated in this study. Depression disorders were defined as K‑CESD-R ≥ 16.
Results
When comparing the patterns of mental health between patients with AAV before and during the pandemic, no change in K‑CESD‑R or K‑POMS subscale scores was observed. Among AAV-related indices, regardless of the pandemic, the short-form 36-item Health Survey (SF-36) mental component score (MCS) and physical component score (PCS) were significantly correlated with K‑CESD‑R and could predict current depressive disorders. When the cut-off of Birmingham vasculitis activity score (BVAS) for depressive disorders was obtained by the receiver operator characteristic curve, it significantly predicted current depressive disorders in patients with AAV during the pandemic, unlike those before the pandemic.
Conclusion
We verified that SF-36 MCS and PCS could predict current depressive disorders, regardless of the pandemic, and furthermore, we demonstrated for the first time that BVAS was a predictor of current depressive disorders in patients with AAV during the pandemic unlike those before the pandemic.
Zusammenfassung
Hintergrund
In der vorliegenden Studie wurden die Häufigkeit und Schwere depressiver Störungen vor und während der durch das „severe acute respiratory syndrome coronavirus type 2“ (SARS-CoV-2) verursachten Pandemie bei Patienten verglichen, die an einer mit antineutrophilen zytoplasmatischen Antikörpern assoziierten Vaskulitis (AAV) erkrankt sind. Dazu wurden die koreanische Version der Center for Epidemiologic Studies Depression Scale-Revised (K-CESD-R) und die koreanische Version des Profile of Mood States (K-POMS) für Depression verwendet; außerdem wurden weitere Prädiktoren für aktuell bestehende depressive Störungen bei den Patienten während der Pandemie ermittelt.
Methoden
Von den 61 Patienten mit AAV, die vor der Pandemie teilnahmen, wurden 8 Patienten an andere Krankenhäuser überwiesen, 3 Patienten starben, und 2 verweigerten die Teilnahme an der Studie. Letztlich nahmen 48 Patienten an der Studie teil. Depressive Störungen wurden definiert als ein Wert für K‑CESD-R ≥ 16.
Ergebnisse
Beim Vergleich der Muster für psychische Gesundheit von Patienten mit AAV vor und während der Pandemie wurde keine Veränderung hinsichtlich der Subskalenwerte für K‑CESD‑R oder K‑POMS festgestellt. Unter den AAV-bezogenen Indizes waren – unabhängig von der Pandemie – der Score für die psychische Komponente (MCS) und die physische Komponente (PCS) des Short-Form 36-Item Health Survey (SF-36) in signifikanter Weise mit der K‑CESD‑R korreliert, sie eigneten sich zur Vorhersage bestehender depressiver Störungen. Bei Ermittlung des Grenzwerts des Birmingham Vasculitis Activity Score (BVAS) für depressive Störungen anhand der „receiver operator characteristic curve“ erwies sich dieser als signifikanter Prädiktor für aktuell bestehende depressive Störungen bei Patienten mit AAV während der Pandemie – im Gegensatz zu vor der Pandemie.
Schlussfolgerung
Es wurde erneut nachgewiesen, dass sich mit dem SF-36-MCS und -PCS aktuell bestehende depressive Störungen vorhersagen ließen – unabhängig von der Pandemie –, und darüber hinaus wurde hier erstmals gezeigt, dass der BVAS ein Prädiktor aktuell bestehender depressiver Störungen bei Patienten mit AAV während der Pandemie im Gegensatz zu vor der Pandemie war.
Similar content being viewed by others
Introduction
Based on the 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides, antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is one of two categories of small vessel vasculitides and is characterised by necrotising vasculitis without definite evidence of immune deposits [1]. AAV consists of three subtypes with different clinical features, microscopic polyangiitis, granulomatosis with polyangiitis, and eosinophilic granulomatosis with polyangiitis [2].
Since AAV may involve almost all organs, studies on organ-specific clinical features, mechanisms, and treatments have been actively conducted [3]. In contrast, although the frequency of depressive disorders has been reported to range between 24.6 and 55% in patients with AAV in a few previous studies [4,5,6], there has not been much interest in psychiatric problems, particularly depressive disorders. In our previous study, we reported that 45.9% of Korean patients with AAV had depressive disorders, based on the Korean version of the Center for Epidemiologic Studies Depression Scale-Revised (K-CESD-R) ≥ 16. Furthermore, we demonstrated that both the short-form 36-item health survey (SF-36) mental component score (MCS) and physical component score (PCS) were negatively correlated with K‑CESD‑R and the Korean version of the Profile of Mood States (K-POMS) depression [7,8,9,10].
Since the first half of 2020, the world has been facing the effects of the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) pandemic; wearing face masks in public places has become compulsory, and maintaining distance between people has become a daily routine. Relative isolation, uncertainty and unpredictability may cause mental health problems in the general population and are closely related to an increase in the frequency of depression [11, 12]. In the same context, it can be assumed that there may be an increase in the frequency and severity of depressive disorders in AAV patients during the pandemic. However, there have been few studies on depressive disorders in patients with AAV during the pandemic. Hence, this study re-included the same patients with AAV who participated in our previous study before the pandemic. In this study, we compared the frequency and severity of depressive disorders using K‑CESD‑R and K‑POMS between patients before and during the pandemic, and it determined significant predictors of current depressive disorders during the pandemic.
Materials and methods
Patients
The same patients with AAV who participated in our previous study before the SARS-CoV‑2 pandemic were asked to volunteer for the present study during the pandemic. The inclusion and exclusion criteria were the same as described in our previous study [7]. Of the 61 patients with AAV who participated before the pandemic, 8 patients were transferred to other hospitals, 3 patients died, and 2 patients refused to participate in the present study. Finally, 48 of the 61 patients were included in this study, and their variables between the two different time points, before and during the pandemic were compared (Fig. 1). All patients were vaccinated according to the government policy, and none was infected with SARS-CoV‑2.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of Severance Hospital (4-2016-0901) and was conducted in accordance with the Declaration of Helsinki. The patients’ written informed consent was obtained from all patients.
Clinical data
The follow-up duration was defined as the period from the first participation to this attendance. AAV-specific indices included SF-36 MCS, SF-36 PCS, Birmingham vasculitis activity score (BVAS), vasculitis damage index [13,14,15]. K‑CESD‑R was evaluated and depression disorders were defined as K‑CESD-R ≥ 16 [8, 9]. K‑POMS was also assessed and presented as the result for six individual item and a total value [10].
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as medians with interquartile ranges, whereas categorical variables are expressed as numbers (percentages). Significant differences between the two categorical variables were analysed using the Χ2 and Fisher’s exact tests. Significant differences between two continuous variables were compared using the Mann–Whitney U test. The correlation coefficient between the two variables was obtained using either the Pearson correlation analysis (r) or the Spearman correlation analysis (r2). The optimal cut-off was extrapolated by performing the receiver operator characteristic (ROC) curve analysis and one value having the maximised sum of sensitivity and specificity was selected. The relative risk (RR) of the cut-off for the high activity of AAV was analysed using contingency tables and the Χ2 test. In principle, P-values less than 0.05 were considered statistically significant. Also, in the comparison analyses (Tables 1 and 2), based on the Bonferroni correction, P-value < 0.0125 is considered statistically significant owing to the four variables compared.
Results
Characteristics of patients with AAV between the two-time points
This study was conducted during the SARS-CoV‑2 pandemic with a median follow-up period of 30.5 months from the previous study before the pandemic. To minimise confounding factors, variables of only 48 patients with AAV, not 61 patients who participated in the previous study, were included. There were no significant differences in demographic data, AAV subtypes, or ANCA positivity between the two time points. Among AAV-specific indices, during the pandemic the median SF-36 PCS was slightly higher than that before the pandemic (66.9 vs. 60.2, P = 0.075), but the difference was not statistically significant. In addition to BVAS, K‑CESD‑R and the frequency of depressive disorder defined as K‑CESD-R ≥ 16 did not differ significantly between the two time points (Table 1, Fig. S1). Clinical manifestations, laboratory results, and administered drugs before and during the pandemic are available in Table S1. In particular, there was no difference in the median glucocorticoid dose (equivalent to prednisolone) between the two time points, which could somewhat exclude the possibility of the relevant role of glucocorticoids in the development of depressive disorders.
Correlation of AAV-specific indices with K-CESD-R and K-POMS subscales in patients with AAV between the two-time points
Patients with AAV at both time points exhibited significant inverse correlations between SF-36 MCS and PCS with K‑CESD‑R, K‑POMS vigour, fatigue, and confusion. In terms of K‑POMS depression, both SF-36 MCS and PCS were significantly correlated with K‑POMS depression before the pandemic; however, only SF-36 PCS exhibited a significant correlation during the pandemic. In addition, a significant correlation between SF-36 PCS and total K‑POMS values before the pandemic disappeared during the pandemic. Conversely, patients with AAV during the pandemic exhibited significant correlations of BVAS with K‑CESR‑D unlike those before the pandemic. However, BVAS had no influence on K‑POMS depression in patients with AAV at either time point (Table 2).
Optimal cut-offs of SF-36 MCS and PCS for depressive disorders and their relative risks during the SARS-CoV-2 pandemic
Only 48 of 61 patients with AAV, who participated in our previous study, were enrolled in this study, and thus, the area under the curve (AUC) of both SF-36 MCS and PCS for depressive disorders, defined as K‑CESD-R ≥ 16 in patients with AAV before the SARS-CoV‑2 pandemic was re-analysed using the ROC curve. The reason was that the results of the ROC curve analysis before the pandemic were not the same as those that were shown in our published paper including 61 patients [7].
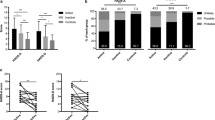
In terms of SF-36 MCS for depressive disorders, patients with AAV during the pandemic had a significant AUC in the ROC analysis (area 0.848), which was comparable to that before the pandemic (area 0.831). When the optimal cut-off of SF-36 MCS for K‑CESD-R ≥ 16 was set as 54.38, the sensitivity and specificity were 96.8 and 64.7%, respectively. When the patients were divided into two groups based on SF-36 MCS ≤ 54.38, depressive disorders were identified more frequently in patients with AAV with SF-36 MCS ≤ 54.38 than those with SF-36 MCS > 54.38 (91.7 vs. 16.7%, P < 0.001). Furthermore, AAV patients with SF-36 MCS ≤ 54.38 exhibited a significantly higher risk for depressive disorders than those with SF-36 MCS > 54.38 (RR 55.000, 95% confidence interval [CI] 5.933, 509.897; Fig. 2).
Cut-offs of SF-36 MCS and PCS for depressive disorders during the SARS-CoV‑2 pandemic. Both SF-36 MCS and PCS exhibited a significant area under the curve in the ROC curve analysis in patients with AAV regardless of the pandemic. AAV patients with SF-36 MCS ≤ 54.38 exhibited a significantly higher risk for depressive disorders than those with SF-36 MCS > 54.38 (RR 55.000), and those with AAV with SF-36 PCS ≤ 62.97 also exhibited a significantly higher risk for depressive disorders than those with SF-36 PCS > 62.97 (RR 11.143). SF-36 the 36-item short form health survey questionnaire, MCS mental component score, PCS physical component score, SARS-CoV‑2 severe acute respiratory syndrome coronavirus type 2, ROC receiver operator characteristic, AAV ANCA-associated vasculitis, ANCA antineutrophil cytoplasmic antibody, RR relative risk, K‑CESD‑R the Korean version of the Center for Epidemiologic Studies Depression Scale-Revised
In terms of SF-36 PCS for depressive disorders, patients with AAV during the pandemic also had a significant AUC in the ROC curve (area 0.776), which was also comparable to that before the pandemic (area 0.804). When the optimal cut-off of SF-36 PCS for K‑CESD-R ≥ 16 was set as 62.97, the sensitivity and specificity were 77.4 and 76.5%, respectively. When the patients were partitioned into two groups based on SF-36 PCS ≤ 62.97, depressive disorders were found more frequently in patients with AAV with SF-36 PCS ≤ 62.97 than those with SF-36 PCS > 62.97 (65.0 vs. 14.3%, P < 0.001). Furthermore, patients with AAV with SF-36 PCS ≤ 62.97 exhibited a significantly higher risk for depressive disorders than those with SF-36 PCS > 62.97 (RR 11.143, 95% CI 2.743, 45.262; Fig. 2).
Optimal cut-off of BVAS for depressive disorders and its relative risk during the SARS-CoV-2 pandemic
In the Spearman correlation analysis, a significant correlation between BVAS and K‑CESD‑R was observed in patients with AAV during the SARS-CoV‑2 pandemic (r2 = 0.347, P = 0.016), whereas, no correlation was observed before the pandemic (r2 = 0.084). Moreover, in terms of BVAS for depressive disorders defined as K‑CESD-R ≥ 16, patients with AAV during the pandemic had a significant AUC (area 0.711). When the optimal cut-off of BVAS for K‑CESD-R ≥ 16 was set to 9.5, the sensitivity and specificity were 41.7 and 93.5%, respectively. When patients with AAV were divided into two groups based on BVAS ≥ 9.5, depressive disorders were found more frequent in patients with AAV with BVAS ≥ 9.5 than in those with BVAS < 9.5 (80.0 vs. 23.7%, P < 0.001). Furthermore, patients with AAV with BVAS ≥ 9.5 exhibited a significantly higher risk for depressive disorders than those with BVAS < 9.5 (RR 12.889, 95% CI 2.307, 72.016; Fig. 3).
Cut-off of BVAS for depressive disorders during the SARS-CoV‑2 pandemic. A significant correlation between BVAS and K‑CESD‑R was observed in patients with AAV during the SARS-CoV‑2 pandemic, unlike those before the pandemic. BVAS exhibited a significant the area under the curve in the ROC curve analysis in patients with AAV during the pandemic. Patients with AAV with BVAS ≥ 9.5 exhibited a significantly higher risk for depressive disorders than those with BVAS < 9.5 (RR 12.889). BVAS Birmingham vasculitis activity score, SARS-CoV‑2 severe acute respiratory syndrome coronavirus type 2, K‑CESD‑R the Korean version of the Center for Epidemiologic Studies Depression Scale-Revised, ROC receiver operator characteristic, AAV ANCA-associated vasculitis, ANCA antineutrophil cytoplasmic antibody, RR relative risk
Discussion
In this study, we drew three important conclusions from the investigation of patients with AAV during the SARS-CoV‑2 pandemic. First, when comparing the patterns of mental health between patients with AAV before and during the pandemic, no change in K‑CESD‑R or K‑POMS subscale scores was observed. Second, regardless of the pandemic, SF-36 MCS and PCS were significantly correlated with K‑CESD‑R and could predict current depressive disorders defined as K‑CESD-R ≥ 16. Third, BVAS was found to be a predictor of current depressive disorders in patients with AAV during the pandemic, unlike those before the pandemic.
It has been reported that during the SARS-CoV‑2 pandemic, mental health deterioration due to the fear of SARS-CoV‑2 infection, limitations in daily life, and socioeconomic difficulties are clearly progressing [16]. According to a recent study, 30.7% of 2288 Korean adult individuals experienced depression during the SARS-CoV‑2 pandemic, highlighting the need for early intervention for mental health issues [17]. Therefore, at the beginning of this study, it was expected that the frequency of depressive disorders in the present study would be significantly higher than that in our previous study. However, when the frequency of depressive disorders defined as K‑CESD-R ≥ 16 was compared between the two time points, patients with AAV during the pandemic did not show a higher frequency of depressive disorders than before the pandemic (35.4 vs. 41.7%). Although it is difficult to elucidate the exact mechanism, it seems that there is an upper limit for the total amount of depression in patients with AAV exposed to chronic inflammation for a long time, unlike in the general population. In addition, within the upper limit for the total amount of depression, it can be assumed that the proportion of effects on the potential for the occurrence of depressive disorders in patients with AAV may vary.
The most important result of our previous study was that the cut-offs of SF-36 MCS and PCS for K‑CESD-R ≥ 16 were determined in patients with AAV, and since we used the cut-offs of SF-36 MCS and PCS that are routinely obtained at every visit, instead of filling out the K‑CESD‑R form, depressive disorders could be estimated in actual clinical practice. Similarly, in this study, the cut-offs of SF-36 MCS and PCS for K‑CESD-R ≥ 16 were also identified, and they could significantly predict current depressive disorders in patients with AAV during the SARS-CoV‑2 pandemic. In real clinical settings, patients with AAV are usually asked to fill out the SF-36 form during every regular visit, but it is not recommended to complete the K‑CESD‑R form. Therefore, even in patients with AAV with subtle depressive symptoms, early detection of depressive disorders based on K‑CESD‑R becomes difficult. At this time, if depressive disorders are screened using SF-36 in patients with AAV, and then K‑CESD‑R is applied to patients with suspected depressive disorders, it is believed that they can be diagnosed and treated early, particularly those that develop during the pandemic [18].
Regarding patients with AAV before the SARS-CoV‑2 pandemic, BVAS was not significantly correlated with SF-36 MCS (r = −0.128, P = 0.385) or SF-36 PCS (r = −0.154, P = 0.295). However, despite the significant correlations of SF-36 PCS with BVAS and K‑CESD‑R, BVAS was not significantly correlated with K‑CESD‑R (r = 0.104; Table 2). Meanwhile, regarding patients with AAV during the pandemic, BVAS was inversely correlated with both SF-36 MCS (r = −0.320, P = 0.001) and SF-36 PCS (r = −0.349, P < 0.001). In addition, as expected, BVAS was significantly correlated with K‑CESD‑R (r = 0.511; Table 2). Summarising the results so far, SF-36 MCS and PCS were inversely correlated with K‑CESD‑R and significantly predicted current depressive disorders in patients with AAV for both before and during the pandemic. However, BVAS exhibited a significant correlation with K‑CESD‑R and predicted depressive disorders only in patients with AAV patients during the pandemic, which arouses interest.
The hypotheses as to how BVAS is significantly linked to the likelihood of depression disorders are as follows. First, given that K‑CESD‑R and the frequency of depressive disorders defined as K‑CESD-R > 16 did not differ between the two time points, the total amount of the potential of the occurrence of depressive disorders was the same, regardless of the pandemic. Second, since BVAS did not differ between the two time points, it cannot be concluded that this discrepancy resulted from an increase in the absolute activity of AAV in patients during the pandemic. Third, there have been reports that the frequency of depressive disorders increased due to the fear of SARS-CoV‑2 infection, limitations in daily life, and socioeconomic difficulties [16]. Therefore, this discrepancy cannot be attributed to a decrease in the influence of the first and second groups of risk factors on the occurrence of depressive disorders. Fourth, it can be reasonably speculated that the potential of the occurrence of depressive disorders was more sensitive to BVAS. Therefore, it was concluded that active therapeutic intervention to lower BVAS of patients with AAV during the pandemic can ultimately reduce the risk of developing depressive disorders.
The effect of depressive disorders based on K‑CESD‑R on SF-36 or BVAS may be greater than the effect of SF-36 or BVAS on K‑CESD‑R. However, even if this is the case, it is impossible to receive the K‑CESD‑R questionnaire from all AAV patients in actual clinical practice. On the other hand, obtaining an SF-36 form and assessing BVAS are currently being performed in clinical practice. Therefore, the gist of this study result is not to reveal the direction in which they affect, but to screen patients who have depressive disorders using SF-36 and BVAS and give them the opportunity to be treated by psychiatric specialists.
The merit of this study was that we verified the clinical utility of SF-36 MCS and PCS in screening for depressive disorders in patients with AAV during the SARS-CoV‑2 pandemic and compared it to that in the same population of patients before the pandemic. In addition, for the first time, we demonstrated that BVAS could predict current depressive disorders in patients with AAV during the pandemic. Including the same patients with AAV who participated in our previous study and comparing the paired clinical data between the two time points based on the pandemic provided dynamic information and aided in overcoming the limitations of the previous study. Moreover, this study provided a method to obtain the cut-offs of parameters for predicting the cross-sectional depressive disorder rather than the fixed cut-offs, which could properly apply the results of this study to patients of different ethnicities and nations. However, this study had several limitations. The small number of patients, which was one of the limitations of the previous study, remained a limitation in this study since only the patients who participated in the previous study were included in the present study. Another limitation of this study is that it failed to actively analyse causes other than AAV that might have contributed to the development of depressive disorders during the SARS-CoV‑2 pandemic, particularly personality disorders or socioeconomic status. Nevertheless, this study has clinical significance in that we clarified that both SF-36 and BVAS could not only estimate current activity of vasculitis but also screen for current depressive disorders in patients with AAV during the pandemic.
Conclusions
We observed no change in K‑CESD‑R or K‑POMS subscale scores in patients with AAV before and during the SARS-CoV‑2 pandemic and those during the pandemic. Nonetheless, we verified that SF-36 MCS and PCS were significantly correlated with K‑CESD‑R and could predict current depressive disorders defined as K‑CESD-R ≥ 16, regardless of the pandemic. In addition, we demonstrated for the first time that BVAS was a predictor of current depressive disorders in patients with AAV during the pandemic.
References
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC et al (2013) 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum 65(1):1–11
Watts R, Lane S, Hanslik T, Hauser T, Hellmich B, Koldingsnes W, Mahr A, Segelmark M, Cohen-Tervaert JW, Scott D (2007) Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides nd polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 66(2):222–227
Kitching AR, Anders HJ, Basu N, Brouwer E, Gordon J, Jayne DR, Kullman J, Lyons PA, Merkel PA, Savage COS et al (2020) ANCA-associated vasculitis. Nat Rev Dis Primers 6(1):71
Hinojosa-Azaola A, Jiménez-González A, Alcocer-Castillejos N (2018) Patient and physician perspectives on the impact of health-related quality of life in Mexican patients with ANCA-associated vasculitis. Rheumatol Int 38(4):631–640
Tomasson G, Boers M, Walsh M, LaValley M, Cuthbertson D, Carette S, Davis JC, Hoffman GS, Khalidi NA, Langford CA et al (2012) Assessment of health-related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res 64(2):273–279
Basu N, McClean A, Harper L, Amft EN, Dhaun N, Luqmani RA, Little MA, Jayne DR et al (2013) Explaining fatigue in ANCA-associated vasculitis. Rheumatology 52(9):1680–1685
Yun JD, Ha J, Kim S, Park HA, Yoo J, Ahn SS, Jung SM, Song JJ, Park YB, Lee SW (2019) Predictor of depressive disorders in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Clin Rheumatol 38(12):3485–3491
Radloff LS (1991) The use of the center for epidemiologic studies depression scale in adolescents and young adults. J Youth Adolesc 20(2):149–166
Fushimi M, Saito S, Shimizu T (2013) Prevalence of depressive symptoms and related factors in Japanese employees as measured by the Center for Epidemiologic Studies Depression Scale (CES-D). Community Ment Health J 49(2):236–242
Norcross JC, Guadagnoli E, Prochaska JO (1984) Factor structure of the Profile of Mood States (POMS): two partial replications. J Clin Psychol 40(5):1270–1277
Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G (2020) Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord 277:5–13
Park KH, Kim AR, Yang MA, Park JH (2021) Differences in multi-faceted lifestyles in response to the COVID-19 pandemic and their association with depression and quality of life of older adults in south korea: a cross-sectional study. Nutrients 13(11):4124
Han CW, Lee EJ, Iwaya T, Kataoka H, Kohzuki M (2004) Development of the Korean version of Short-Form 36-Item Health Survey: health related QOL of healthy elderly people and elderly patients in Korea. Tohoku J Exp Med 203(3):189–194
Mukhtyar C, Lee R, Brown D, Carruthers D, Dasgupta B, Dubey S, Flossmann O, Hall C, Hollywood J, Jayne D et al (2009) Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 68(12):1827–1832
Bhamra K, Luqmani R (2012) Damage assessment in ANCA-associated vasculitis. Curr Rheumatol Rep 14(6):494–500
Park KH, Kim AR, Yang MA, Lim SJ, Park JH (2021) Impact of the COVID-19 pandemic on the lifestyle, mental health, and quality of life of adults in South Korea. PLoS ONE 16(2):e247970
Kim DM, Bang YR, Kim JH, Park JH (2021) The prevalence of depression, anxiety and associated factors among the general public during COVID-19 pandemic: a cross-sectional study in Korea. J Korean Med Sci 36(29):e214
Kristjánsdóttir J, Olsson GI, Sundelin C, Naessen T (2011) Could SF-36 be used as a screening instrument for depression in a Swedish youth population? Scand J Caring Sci 25(2):262–268
Funding
This research was supported by a faculty research grant of Yonsei University College of Medicine (6-2019-0184) and a grant from the Korea Health Technology Research and Development Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (HI14C1324).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JDY, JHL and SWL. The first draft of the manuscript was written by JDY and SWL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
J.-D. Yun, J.H. Lee, J.Y. Pyo, S.S. Ahn, J.J. Song, Y.-B. Park and S.-W. Lee declare that they have no competing interests.
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Redaktion
Ulf Müller-Ladner, Bad Nauheim
Uwe Lange, Bad Nauheim
Availability of data and material
Datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.

Scan QR code & read article online
Supplementary Information
Figure S1
Comparison of K‑CESD‑R and BVAS between AAV patients before and during the SARS-CoV‑2 pandemic. K‑CESD‑R The Korean version of the Center for Epidemiologic Studies Depression Scale-Revised; BVAS Birmingham vasculitis activity score; AAV antineutrophil cytoplasmic antibody-associated vasculitis; SARS-CoV‑2 the severe acute respiratory syndrome coronavirus-2
Table S1
Characteristics of AAV patients between before and during the SARS-CoV‑2 pandemic
Rights and permissions
About this article
Cite this article
Yun, JD., Lee, J.H., Pyo, J.Y. et al. Birmingham vasculitis activity score and the short form 36-item health survey predict current depressive disorders in patients with antineutrophil cytoplasmic antibody-associated vasculitis during the SARS-CoV-2 pandemic. Z Rheumatol 83 (Suppl 1), 222–229 (2024). https://doi.org/10.1007/s00393-022-01233-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-022-01233-1
Keywords
- Antineutrophil cytoplasmic antibody-associated vasculitis
- Severe acute respiratory syndrome coronavirus‑2
- Activity
- Function
- Depression