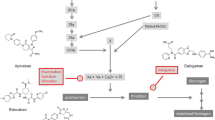
Abstract
Background
The effects of left atrial appendage (LAA) occlusion compared to non-vitamin K antagonist oral anticoagulant (NOAC) therapy in patients with atrial fibrillation (AF) remain unknown.
Aims
We aimed to evaluate the outcomes in patients with AF who received LAA occlusion vs. NOAC therapy.
Methods
We utilised data from TriNetX which is a global federated health research network currently containing data for 88.5 million patients. ICD-10 codes were employed to identify AF patients treated with either LAA occlusion or NOAC between 1st December 2010 and 17th January 2019. Clinical outcomes of interest were analysed up to 2 years.
Results
108,697 patients were included. Patients who underwent LAA occlusion were younger, more likely to be white Caucasian and male, had a greater incidence of comorbidities, and were less likely to be prescribed other cardiovascular medications. Using propensity score matching, the risk of all-cause mortality was significantly lower among patients who received LAA occlusion compared to NOAC therapy [1.51% vs. 5.60%, RR 0.27 (95% CI 0.14–0.54)], but there were no statistical differences in the composite thrombotic or thromboembolic events [8.17% vs. 7.72%, RR 1.06 (95% CI 0.73–1.53)], ischaemic stroke or TIA [4.69% vs. 5.45%, RR 0.86 (95% CI 0.54–1.38)], venous thromboembolism [1.66% vs. 1.51%, RR 1.10 (95% CI 0.47–2.57)] and intracranial haemorrhage [1.51% vs. 1.51%, RR 1.00 (95% CI 0.42–2.39)].
Conclusion
Overall, LAA occlusion might be a suitable alternative to NOAC therapy for stroke prevention in patients with AF.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin K antagonists (VKAs), such as warfarin, have traditionally been used to prevent thromboembolic complications in patients with atrial fibrillation (AF). However, warfarin is limited by a narrow therapeutic window, an increased risk of bleeding, and possible interactions with other drugs and food. Over the past decade, non-vitamin K antagonist oral anticoagulants (NOACs) have been shown in several landmark randomised controlled trials (RCTs) to be superior to warfarin, with comparable efficacy for stroke prevention but reduced risk of serious bleeding [1,2,3,4,5]. Nonetheless, there remains a proportion of patients who are unsuitable for anticoagulation due to conditions that predispose to a very high-risk of life-threatening bleeding, severe side effects or pregnancy (1st and 3rd trimester).
More recently, left atrial appendage (LAA) occlusion has emerged as a potential alternative therapy for stroke prevention in AF [6]. Findings from the National Cardiovascular Data Registry LAA occlusion registry of 38,158 procedures performed between January 2016 and December 2018 showed that the procedure was associated with a success rate of 98.1% to achieve a less than 5 mm leak, while maintaining a low incidence of major in-hospital adverse events (2.2%) [7]. Furthermore, LAA occlusion may result in better long-term outcomes compared to warfarin [8]. However, there are limited studies comparing the effects of LAA occlusion to NOAC therapy. To date, the PRAGUE-17 trial remains the only prospective RCT that has addressed this topic [9]. Herein, we aimed to evaluate the outcomes in patients with AF who received LAA occlusion vs. NOAC therapy.
Methods
In this study, we used data from TriNetX, a global federated health research network with real-time updates of anonymised electronic medical records, predominantly in the United States. The network currently comprised 66 health-care organisations, including academic medical centres, speciality physician practices and community hospitals, and contains data for around 88.5 million patients across 11 countries. Further details about TriNetX processes and standardisation of data are in the Supplementary Materials.
We performed a search using ICD-10 codes (details in Supplementary Materials) on the 26th of January 2021 for patients with AF who were treated with either LAA occlusion or NOAC between 1st of December 2010 and 17th of January 2019. All patients who were aged over 18 years and received either surgical/catheter LAA occlusion or NOAC therapy alone were included. Exclusion criteria were rheumatic heart disease and acute rheumatic fever.
Data on baseline demographics, comorbidities (e.g. hypertension, coronary artery disease, diabetes mellitus, heart failure, previous stroke, peripheral vascular disease, prior gastrointestinal haemorrhage and prior intracerebral haemorrhage) and medication use (e.g. anticoagulants, antiplatelets, beta-blockers, calcium channel blockers, anti-arrhythmic drugs, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and diuretics) were collected.
Clinical outcomes of interest were all-cause mortality, composite thrombotic and thromboembolic events, ischaemic stroke or transient ischaemic attack (TIA), venous thromboembolism and intracranial haemorrhage. These were recorded from 30 days after treatment until a pre-specified follow-up duration of 2 years and defined using ICD-10 codes.
As a federated network, research using TriNetX does not require ethical approval. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating health-care organisations and their individual contribution to each dataset are not disclosed. The TriNetX platform only uses aggregated counts and statistical summaries of de-identified information. No protected health information or personal data is made available.
Statistical analysis
Continuous variables were expressed as mean and standard deviation (SD), and tested for differences with independent-sample t test. Categorical variables were expressed as absolute frequencies and percentages, and tested for differences with Chi-squared test. Propensity score matching (PSM) in a 1:1 ratio was performed using logistic regression with nearest-neighbour matching at a tolerance level of 0.01 and difference between propensity scores of equal or less than 0.1 (i.e. caliper) for age, sex, race, hypertension, hypercholesterolaemia, coronary artery disease, diabetes mellitus, heart failure, chronic obstructive pulmonary disease, previous stroke, peripheral vascular disease, prior gastrointestinal haemorrhage, prior intracerebral haemorrhage, anticoagulant use, antiplatelet therapy, beta-blocker, calcium channel blocker use, anti-arrhythmic drug therapy, angiotensin-converting enzyme inhibitor use, angiotensin receptor blocker use and diuretic therapy. Covariate balance between groups was assessed using standardised mean differences, with a value below 0.1 indicating minimal differences between groups. Plots of Kaplan–Meier curves for study outcomes were created and survival distributions were assessed using log-rank test. Relative risk (RR) with 95% confidence intervals (CIs) was calculated. To validate our findings, an additional analysis was performed to compare the effects of LAA occlusion vs. VKA therapy in a separate subgroup of PSM patients using the method described above. No imputations were made for missing data. All p values were two-sided, and the significance level was set at 0.05. Statistical analysis was performed using the TriNetX Analytics function in the online research platform.
Results
We included 108,697 patients with AF who were treated with LAA occlusion (n = 699) or NOAC therapy (n = 107,998). Compared to patients on NOAC therapy, those who received treatment with LAA occlusion were younger, more likely to be white Caucasian and male, and had a greater incidence of comorbidities including hypertension, hypercholesterolaemia, coronary artery disease, diabetes mellitus, heart failure, chronic obstructive pulmonary disease, previous stroke, peripheral vascular disease, prior gastrointestinal haemorrhage and prior intracerebral haemorrhage (Table 1). Patients in the LAA occlusion group were less likely to be prescribed other cardiovascular medications such as beta-blockers, calcium channel blockers, anti-arrhythmic drugs, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and diuretics. The most commonly used NOAC was apixaban, followed by rivaroxaban, dabigatran and edoxaban. After PSM, there were a total of 1,322 patients in both groups with comparable baseline characteristics (Table 2).
Clinical outcomes before PSM (unadjusted)
The follow-up duration was comparable between both groups. At 2 years, the risk of all-cause mortality was significantly lower in the LAA occlusion group (1.43%) compared to the NOAC group (4.41%) (sTable 1). There were no significant differences in terms of the risk of composite thrombotic or thromboembolic events, or ischaemic stroke or TIA. The risk of venous thromboembolism was reduced with LAA occlusion though these patients were at an increased risk of intracranial haemorrhage.
Clinical outcomes after PSM
At 2 years, the risk of all-cause mortality was significantly lower among patients who received LAA occlusion compared to NOAC therapy [1.51% vs. 5.60%, RR 0.27 (95% CI 0.14–0.54)], with no statistical difference in terms of the composite thrombotic or thromboembolic events [8.17% vs. 7.72%, RR 1.06 (95% CI 0.73–1.53)], ischaemic stroke or TIA [4.69% vs. 5.45%, RR 0.86 (95% CI 0.54–1.38)], venous thromboembolism [1.66% vs. 1.51%, RR 1.10 (95% CI 0.47–2.57)] and intracranial haemorrhage [1.51% vs. 1.51%, RR 1.00 (95% CI 0.42–2.39)] (Table 3). Kaplan–Meier survival analysis curves with the respective log-rank values are shown in sFigure 1.
Analysis of PSM cohorts with LAA occlusion vs. VKA
In a separate analysis of PSM cohorts with LAA occlusion vs. VKA (sTable 2), we found that LAA occlusion was associated with a significant reduction in all-cause mortality [1.46% vs. 6.85%, RR 0.21 (95% CI 0.11–0.42)], composite thrombotic or thromboembolic events [7.87% vs. 12.97%, RR 0.61 (95% CI 0.44–0.84)], and ischaemic stroke or TIA [4.52% vs. 7.14%, RR 0.63 (95% CI 0.41–0.98)] (Table 4). There were no statistical differences between the groups for venous thromboembolism [1.60% vs. 3.06%, RR 0.52 (95% CI 0.25–1.08)] and intracranial haemorrhage [1.46% vs. 1.75%, RR 0.83 (95% CI 0.36–1.92)].
Discussion
In this study, we compared the long-term outcomes of LAA occlusion against NOAC therapy in a large cohort of patients with AF across several health-care organisations, mainly from the USA. The major findings were that patients with AF who received LAA occlusion over NOAC therapy: (1) were younger, more likely to be white Caucasian and male with a greater incidence of comorbidities; (2) were prescribed less cardiovascular medications; (3) had a significantly reduced risk of all-cause mortality at 2-year follow-up; (4) had no statistical difference in the long-term outcomes of the composite of thrombotic or thromboembolic events, ischaemic stroke or TIA, venous thromboembolism, and intracranial haemorrhage. We also demonstrated that LAA occlusion was associated with a reduction in all-cause mortality compared to VKA. Moreover, there was a significant benefit of LAA occlusion over VKA, in terms of the composite thrombotic or thromboembolic events, and ischaemic stroke or TIA which was not observed in the comparison between LAA occlusion and NOAC therapy.
The baseline characteristics of patients who underwent LAA occlusion in this study were similar to that of both the PROTECT AF and PREVAIL RCTs which recruited patients who were eligible for warfarin [10, 11], indicating appropriate patient selection in this real-world cohort. Overall, there was a very low incidence of prior major bleeding in both groups, especially intracranial haemorrhage. In contrast, the incidence of prior major bleeding was much higher (> 70%) in other real-world studies that were limited to the use of LAA occlusion in patients who had contraindications to systemic anticoagulation [12, 13]. This may partly explain the low rates of mortality at 2 years. Despite a greater burden of comorbidities, we observed that patients who received LAA occlusion were prescribed far fewer cardiovascular medications. This may suggest a degree of intolerance to medical therapy, thereby contributing to the indication for LAA occlusion in the first instance. However, the possibility of residual confounders cannot be excluded with this observational study design.
We demonstrated that patients with AF who received LAA occlusion had a significantly reduced risk of all-cause mortality compared to those treated with NOAC. Initial differences in the risk of venous thromboembolism and intracranial haemorrhage between the groups were due to disparities in baseline characteristics, or confounding by indication as those perceived to be at higher risk of intracranial haemorrhage are often referred for LAA occlusion. Furthermore, both groups had a comparable risk of thrombotic and thromboembolic complications, and ischaemic stroke or TIA. The exact mechanisms in which LAA occlusion offers prognostic mortality benefit remains undetermined and could merely be a chance finding. Unfortunately, we were unable to delineate between cardiovascular and non-cardiovascular causes of death.
A study of high-risk patients with AF enrolled in the Amulet Observation Registry reported that patients who were treated with LAA occlusion had a significantly lower risk of the composite outcome of ischaemic stroke, major bleeding or all-cause mortality compared to those who received NOAC therapy over a 2-year follow-up period [14]. In contrast, but similar to our findings, the PRAGUE-17 trial which remains the only randomised controlled trial to directly compare the effects of LAA occlusion vs. NOAC therapy, demonstrated no significant difference in the composite outcome of stroke/TIA and clinically significant bleeding between either treatment [9]. This study was limited to patients with a history of major bleeding, resistant stroke, or moderate- to high-risk profile by CHA2DS2-VASc and HAS-BLED scores. A network meta-analysis of 14 studies with 246,005 patients also found no significant differences in outcomes between LAA occlusion and NOAC therapy but relied on indirect comparisons to arrive at that conclusion [15]. Further studies are needed to investigate the possible prognostic benefit offered by LAA occlusion found in our study. In this regard, the CATALYST (NCT04226547), Occlusion-AF (NCT03642509) and CLOSURE-AF (NCT03463317) trials may provide useful information but are not due to be completed until the end of this decade.
Apart from all-cause mortality, long-term outcomes in this cohort were comparable to other studies. In PROTECT AF, the observed rate of all-cause mortality was 3.0 (95% CI 1.9–4.5) per 100 patient-years (PYs), ischaemic stroke was 2.2 (95% CI 1.2–3.5) per 100 PYs and haemorrhagic stroke was 0.1 (95% CI 0–0.5) per 100 PYs in the intervention arm compared to 4.8 (95% CI 2.8–7.1) per 100 PYs, 1.6 (95% CI 0.6–3.0) per 100 PYs and 1.6 (95% CI 0.6–3.1) per 100 PYs in the warfarin arm, respectively [10]. During the 2-year follow-up, the EWOLUTION registry found that LAA occlusion was associated with an all-cause mortality of 16.4% (95% CI 13.8–19.3%), stroke rate of 1.3 (95% CI 0.8–1.9) per 100 PYs and major bleeding rate of 2.7 (95% CI 2.0–3.6) per 100 PYs [16]. A real-world observational study of LAA occlusion by Tzikas et al. reported 1-year outcomes in terms of all-cause mortality, systemic thromboembolism and major bleeding of 4.3%, 2.3% and 2.1%, respectively [17]. In the ASAP study, the annual risks of all-cause mortality, ischaemic stroke and haemorrhagic stroke were 5.0%, 1.7% and 0.6%, respectively [18].
Limitations
There are several limitations to this study. Much of the collected data were based on ICD codes from electronic medical records which may vary by patient characteristics and between different health-care organisations [19]. There was also likely a degree of selection bias as we found that patients who received LAA occlusion had a greater incidence of comorbidities. To account for this, we used statistical adjustments and PSM for known confounders. However, we cannot exclude the possibility of residual confounders. The incidence of major bleeding prior to LAA occlusion was low in this cohort and therefore our results may not be applicable to such patients. Furthermore, we did not have data relating to the indication for LAA occlusion, post-LAA occlusion therapy and exact cause of death. As our cohort of patients were comprised of predominantly white Caucasian males, the findings may not be generalisable to the wider population.
Conclusions
Overall, LAA occlusion might be a suitable alternative to NOAC therapy for stroke prevention in low-risk patients with AF and appears to be associated with good long-term outcomes. However, appropriate patient selection remains an integral aspect of this treatment. Further studies are needed to confirm our findings.
Code availability
Not applicable.
References
Granger CB, Alexander JH, McMurray JJV et al (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365:981–992. https://doi.org/10.1056/NEJMoa1107039
Connolly SJ, Ezekowitz MD, Yusuf S et al (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361:1139–1151. https://doi.org/10.1056/NEJMoa0905561
Patel MR, Mahaffey KW, Garg J et al (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365:883–891. https://doi.org/10.1056/NEJMoa1009638
Giugliano RP, Ruff CT, Braunwald E et al (2013) Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369:2093–2104. https://doi.org/10.1056/NEJMoa1310907
Gomez-Outes A, Terleira-Fernandez AI, Calvo-Rojas G et al (2013) Dabigatran, rivaroxaban, or apixaban versus warfarin in patients with nonvalvular atrial fibrillation: a systematic review and meta-analysis of subgroups. Thrombosis 2013:640723. https://doi.org/10.1155/2013/640723
Ding WY, Mandrola J, Gupta D (2020) Left atrial appendage occlusion: past, present and future. Thromb Haemost 120:1484–1491. https://doi.org/10.1055/s-0040-1714654
Freeman JV, Varosy P, Price MJ et al (2020) The NCDR left atrial appendage occlusion registry. J Am Coll Cardiol 75:1503–1518. https://doi.org/10.1016/j.jacc.2019.12.040
Holmes DRJ, Doshi SK, Kar S et al (2015) Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol 65:2614–2623. https://doi.org/10.1016/j.jacc.2015.04.025
Osmancik P, Herman D, Neuzil P et al (2020) Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 75:3122–3135. https://doi.org/10.1016/j.jacc.2020.04.067
Holmes DR, Reddy VY, Turi ZG et al (2009) Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 374:534–542. https://doi.org/10.1016/S0140-6736(09)61343-X
Holmes DRJ, Kar S, Price MJ et al (2014) Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 64:1–12. https://doi.org/10.1016/j.jacc.2014.04.029
Willits I, Kim K, Sam U, et al (2019) Commissioning through evaluation (CtE) percutaneous occlusion of the left atrial appendage in non-valvular atrial fibrillation for the prevention of thromboembolism (LAAO). https://www.england.nhs.uk/wp-content/uploads/2018/07/1692-left-atrialappendage-occlusion.pdf
Williams T, Alsanjari O, Parker J et al (2018) Day-case percutaneous left atrial appendage occlusion-safety and efficacy. Catheter Cardiovasc Interv 92:1439–1443. https://doi.org/10.1002/ccd.27791
Nielsen-Kudsk JE, Korsholm K, Damgaard D et al (2021) Clinical outcomes associated with left atrial appendage occlusion versus direct oral anticoagulation in atrial fibrillation. JACC Cardiovasc Interv 14:69–78. https://doi.org/10.1016/j.jcin.2020.09.051
Koifman E, Lipinski MJ, Escarcega RO et al (2016) Comparison of Watchman device with new oral anti-coagulants in patients with atrial fibrillation: a network meta-analysis. Int J Cardiol 205:17–22. https://doi.org/10.1016/j.ijcard.2015.11.181
Boersma LV, Ince H, Kische S et al (2019) Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the Watchman left atrial appendage closure technology. Circ Arrhythm Electrophysiol 12:e006841. https://doi.org/10.1161/CIRCEP.118.006841
Tzikas A, Shakir S, Gafoor S et al (2016) Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the Amplatzer Cardiac Plug. EuroIntervention 11:1170–1179. https://doi.org/10.4244/EIJY15M01_06
Reddy VY, Mobius-Winkler S, Miller MA et al (2013) Left atrial appendage closure with the Watchman device in patients with a contraindication for oral anticoagulation: the ASAP study (ASA plavix feasibility study with Watchman left atrial appendage closure technology). J Am Coll Cardiol 61:2551–2556. https://doi.org/10.1016/j.jacc.2013.03.035
Chong WF, Ding YY, Heng BH (2011) A comparison of comorbidities obtained from hospital administrative data and medical charts in older patients with pneumonia. BMC Health Serv Res 11:105. https://doi.org/10.1186/1472-6963-11-105
Acknowledgements
None.
Funding
WYD, EF-E and PU: none declared. JMRC: grant from Sociedad Española de Trombosis y Hemostasia (grant for short international training stays 2020) and the First Contact Initiative Grant 2020 from the European Society of Cardiology Council on Basic Cardiovascular Science. DG: speaker for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Biosense Webster and Boston Scientific; proctor for Abbott; research grants from Medtronic, Biosense Webster and Boston Scientific. FM: research grants from Boehringer Ingelheim, Bayer and Ferrer; consultant and speaker for Boehringer-Ingelhemim, Astra Zeneca and Bayer. GYHL: consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo. No fees were directly received personally.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
As a federated network, research using TriNetX does not require ethical approval. To comply with legal frameworks and ethical guidelines guarding against data re-identification, the identity of participating health-care organisations and their individual contribution to each dataset are not disclosed. The TriNetX platform only uses aggregated counts and statistical summaries of de-identified information. No protected health information or personal data is made available.
Consent to participate
See above.
Consent for publication
See above.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ding, W.Y., Rivera-Caravaca, J.M., Fazio-Eynullayeva, E. et al. Outcomes of left atrial appendage occlusion vs. non-vitamin K antagonist oral anticoagulants in atrial fibrillation. Clin Res Cardiol 111, 1040–1047 (2022). https://doi.org/10.1007/s00392-021-01983-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-021-01983-z