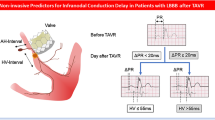
Abstract
Background
Transcatheter aortic valve implantation (TAVI) has been developed to minimize operative morbidity and mortality in high-risk symptomatic patients unfit for open surgery. With the proximity of the aortic valve annulus to the conduction system there is, however, an unknown risk of conduction disturbances necessitating monitoring and often cardiac pacing.
Materials and methods
We enrolled 50 consecutive patients from January 2007 to 2008 in our prospective evaluation of conduction disturbances measured by surface and intracardiac ECG recordings. Baseline parameters, procedural characteristics as well as twelve-lead surface ECG and intracardiac conduction times were revealed pre-interventionally, after TAVI and at 7-day follow-up.
Results
TAVI was performed successfully in all patients. During 7 days of follow-up the rate for first-degree AV block raised from 14% at baseline to 44% at day 7 (p < 0.001), while rates for type II second- and third-degree were 0 versus 8% (p < 0.001) and 0 versus 12% (p < 0.001), respectively. Similarly, the prevalence of new left bundle branch block (LBBB) rose from 2 to 54% (p < 0.001). Intracardiac measurements revealed a prolongation of both AH and HV interval from 123.7 ± 41.6 to 136.6 ± 40.5 ms (p < 0.001) and from 54.8 ± 11.7 to 71.4 ± 20.0 ms (p < 0.001), respectively. Pacemaker implantation at a mean follow-up of 4.8 ± 1.2 days was subsequently performed in 23 patients (46%) due to complete AV block (12%) and type II second-degree AV block (8%) while another 13 patients (26%) received a pacemaker for the combination of new LBBB with marked HV prolongation. The high rate of first-degree AV block was primarily driven by an increase in HV interval.
Conclusion
Cardiac conduction disturbances were common in the early experience with CoreValve implantation necessitating close surveillance for at least 1 week.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rising life expectancy results in an increase in degenerative and neoplastic diseases. Population-based observational studies have revealed that 1–2% of patients over 65 years have moderate to severe aortic stenosis [1]. Current guidelines consider aortic valve replacement as a class I indication for symptomatic patients [2, 3], facing, however, the fact that one-third of patients are considered to have an unacceptably high risk for open surgery [4]. Current treatment options for those patients include medical treatment and percutaneous balloon aortic valvuloplasty, although neither has been shown to reduce long-term mortality of medically treated patients with symptomatic aortic stenosis with a 1- and 5-year survival of 60 and 32%, respectively, and only minor short-term benefits were reported after balloon aortic valvuloplasty [5–7]. Transcatheter aortic valve implantation (TAVI) has recently been developed to minimize surgical risk in high-risk patients with severe symptomatic aortic stenosis who are refused for conventional open aortic valve replacement. With the anatomical proximity of the conduction system to the aortic annulus there is potential to develop conduction disorders in up to 6% even after conventional surgical aortic valve replacement [8, 9]. Initial experience with TAVI reported complete AV block and pacemaker requirement in 5.7–42.5% [10–17]. Better prediction of pacemaker requirement would be of considerable benefit in patients undergoing TAVI with respect to potential need and duration of postoperative monitoring. We examined both incidence and characteristics of conduction disorders periinterventionally and during in-hospital follow-up period after TAVI measured by surface and intracardiac ECG recordings.
Methods
Patients
Between January 2007 and 2008, 50 consecutive patients who underwent TAVI using the third-generation percutaneous self-expanding CoreValve prosthesis (Medtronic, Minneapolis, MN, USA) were identified for this study. The criteria for inclusion and exclusion to the TAVI procedure have been described elsewhere [10, 11]. In brief, patients were included with echocardiographic measurements demonstrating severe native valvular aortic stenosis with an area <1 cm2, or <0.6 cm2/m2 regardless of adjunct regurgitation; a diameter of the basal orifice of the stenosed valve between 20 and 27 mm; and a diameter at the sinotubular junction ≤43 mm. Most importantly, all patients were considered unfit for open surgery with a EuroSCORE ≥20% [10, 11, 18]. TAVI was suggested in agreement between a cardiac surgeon and both, a clinical and interventional cardiologist; patient’s or referring physician’s preference was not relevant. Pacemaker implantation at follow-up was considered as indicated in case of complete AV block, type II second-degree AV block, and in presence of new LBBB in combination with HV prolongation to ≥75 ms.
Procedure
Details of the implantation procedures have been described elsewhere [10–17]. In brief, all patients were operated in a hybrid interventional suite under general anesthesia to assure stable hemodynamics and minimize patient movement during valve implantation. TAVI was performed via femoral access under fluoroscopic imaging. The aortic valve was initially dilated using a standard valvuloplasty balloon with a nominal diameter similar to the aortic valve and followed by CoreValve insertion [10, 11].
In each patient, prior to TAVI and aside a right ventricular bipolar pacing lead (Pacel™, St. Jude Medical, St Paul, MN, USA), a 6F quadripolar electrode catheter with ring electrodes (5-mm interpolar distance) (Webster D™, Biosense Webster, Diamond, USA) was introduced and advanced to the His bundle. A 6F quadripolar electrode catheter (Soloist™, Medtronic, Minneapolis, MN, USA) was advanced to the right atrium and another to the right ventricle to record a bipolar electrogram and for programmed atrial and ventricular stimulation. The access sites for all electrophysiologic electrode catheters were the femoral veins. With such instrumentation the sinus node recovery time (SNRT), corrected sinus node recovery time (c-SNRT), antegrade and retrograde effective refractory period (ERP) of the AV node, as well as intracardiac conduction times (atrium to His and His to ventricle time; AH and HV interval) were assessed. The rationale to measure SNRT was the observations of sinus node arrest in a single patient receiving a CoreValve prior this study. Thus, we wanted to avoid overseeing sinus node pathology in this elderly patient suffering from a high comorbidity index. All measurements were done on an Axiom Sensis™ (Siemens, Erlangen, Germany) electrophysiology workstation. The AV nodal ERP was measured by introducing a single extrastimulus (S2) after a drive train of 8 stimuli at a fixed rate (S1) (600 ms), at which time the S1–S2 interval is decreased until the S2 impulse does not conduct to the His bundle. To assess conduction disorders, patients were attached to uninterrupted ECG monitoring using the Philips monitoring system (IntelliVue™, Best, The Netherlands) that is installed at our ICU/IMC unit. All patients were prophylactically given a temporary pacemaker via the existing femoral venous access; with VVI mode the active pacing was 60 bpm for at least 24 h.
Statistical methods
All data were processed using the SPSS statistical package for windows, release 16.0 (Chicago, IL, USA). The descriptive statistical characteristics for quantitative parameters are listed as numbers (n), arithmetic mean (mean), median (med), minimum (min), maximum (max), and relative frequency (%). The Fisher exact test and χ2-test were used to compare proportions. A normal distribution of differences was confirmed by the Kolmogorov–Smirnov test; in presence of normally distributed data paired-sample t testing was used while with non-normal distribution the Wilcoxon rank test was required. Differences were considered significant at a probability value of p < 0.05.
Results
The analysis included 22 men and 28 women with a mean age of 81.5 ± 6.8 years. All patients had qualified for TAVI according to recent recommendations [10, 11]. Clinical symptoms included dyspnoea (56%), angina (42%), syncope (22%) as well as heart failure (38%) (Table 1). The logistic EuroSCORE operative mortality estimate was 23.0 ± 17.5% while 4 patients (8%) were classified inoperable because of porcelain aorta. Echocardiographic mean pressure across the aortic valve was 55 ± 15.4 mmHg with an aortic valve area of 0.7 ± 0.2 cm2 measured by planimetry during transesophageal echocardiography.
TAVI required a mean procedural and fluoroscopy time of 109.6 ± 36.4 min and 14.1 ± 1.6 min, respectively, and was successfully performed in all patients followed by an intensive care and hospital stay of 2.2 ± 2.8 and 13.8 ± 9.3 days. The placement of electrode catheters throughout the procedure resulted in an additional fluoroscopy time of 2.5 min. Six patients suffered from intraprocedural circulatory depression and required intermittent intravenous catecholamines; one patient required 1 DC shock for ventricular fibrillation induced by wire irritation. Postinterventional aortography revealed a mean aortic insufficiency grade of 1.2 ± 0.58 (Table 2).
Sinus rhythm was documented at baseline in 39 (78%) and atrial fibrillation in 9 (18%); five patients (10%) had previously implanted pacemakers due to bradyarrhythmia (n = 3; 6%) and sino-atrial block (n = 2; 4%). Seven patients (14%) had first-degree AV block before TAVI, which increased to 22 (44%) (p < 0.001) at 7-day follow-up. Similarly, newly developed second-degree AV block (all of them being type II blocks) was present in 4 (8%) at follow-up (p < 0.001). LBBB, present in 1 patient (2%) prior to TAVI, was documented at the time of post-procedural electrogram in 20 patients (40%) eventually rising to 27 (54%) at 7-day follow-up (p < 0.001). The two patients with pre-existing left anterior hemiblock developed complete left bundle branch block and the two patients with pre-existing right bundle branch block emerged with complete AV block after placement of the CoreValve prosthesis. All four patients received permanent pacemaker due to either complete AV block or markedly prolonged HV conduction.
Of the 22 patients suffering from first-degree AV block, intracardiac measurements revealed 18 cases of HV prolongation (13 patients with a prolongation to ≥75 ms and 5 patients with a prolongation up to 75 ms) while 8 had AH prolongation without progression to complete heart block and thus not necessitating a permanent pacemaker. Similarly, five patients with HV prolongation to values lower than 75 ms (2 patients with additional new LBBB) received no pacemaker and had no higher degree conduction abnormality during observation. Baseline AH and HV intervals increased from 123.7 ± 41.6 and 54.8 ± 11.7 ms to 136.6 ± 40.5 and 71.4 ± 20.0 ms at 7-day follow-up without any significant change in SNRT, c-SNRT (Table 3). Any increasing intracardiac conduction time was irreversible (Figs. 1, 2).
At 7 days, complete heart block with pacing was present in 6 cases (12%), while another 4 patients (8%) received a pacemaker for type II second-degree AV block and further 13 patients (26%) for the combination of new LBBB with an increase in AV conduction time, especially the HV intervals ≥75 ms (dual chamber pacing in 15 and single chamber pacing in 8 patients). All conduction abnormalities in patients receiving a pacemaker were due to delay or block in infra-His region. Pacemakers were implanted at 4.8 ± 1.2 days of follow-up. None of the five patients with previously implanted pacemakers experienced additional procedure-related pacemaker indication after TAVI.
Discussion
Aortic valve disease has been associated with cardiac conduction system disease as aortic stenosis and insufficiency have been associated with both prolonged AV conduction times and higher degrees of AV block [19–21]. Due to the vicinity of aortic valve and AV node as well as His bundle, AV block is a common complication of conventional surgical aortic valve replacement and has been described in up to 6% [8, 9]. New LBBB after surgical valve replacement is even more common and has been reported in 18% [8, 9]. Furthermore, development of new LBBB after surgical aortic valve replacement is associated with higher rates of complete AV block, syncope, and sudden cardiac arrest at long term [8, 9, 22]. Such conduction disturbances are presumed to result from surgical trauma to the cardiac conduction tissue during debridement of the calcified annulus [8, 9, 22].
TAVI is a relatively new alternative to conventional surgical valve replacement in high-risk patients not eligible for open surgery [10, 11]. There are only few data regarding the incidence of early conduction disorders after TAVI. The incidence of permanent pacemaker implantation after TAVI with the CoreValve system has been reported in 20–42.5%, and that of a new LBBB in 50–70% [12–15, 23]. In the study by Marcheix et al. [23] 30% of patients required pacemaker implantation due to persistent AV block [23], whereas Zahn et al. [12] reported a permanent pacemaker rate of 42.5% in the German Transcatheter Aortic Valve Intervention Registry [12]. Different rates of pacemaker implantation might be due to different indications for pacing (e.g. complete AV block, new LBBB, prolonged AV conduction). A comparison of hard endpoints like high-grade AV block would be more convincing. Other reasons for different pacemaker implantation rates might be the learning curve with high implantation techniques resulting in less compromise of the compact AV node [23–27].
Although several reports describe changes in surface ECG, our study is the first to note intracardiac conduction abnormalities for better discriminating new ECG changes on surface ECG. Interestingly, the evolution to complete AV block and to LBBB took place over an observation period of 7 days. Similarly, PQ interval and QRS duration, as well as AH and HV intervals prolonged and resulted in a rate of permanent pacemakers of 46%. The AH interval is considerably affected by patient’s autonomic state when TAVI procedure is performed under general anesthesia. However, the main changes during TAVI results in HV conduction. Scheinman et al. [24] have shown that patients with an HV interval greater than 100 ms are at high risk to develop complete AV block. Therefore, the possibility of progression of LBBB to complete AV block should always be considered [22, 24, 25] and may explain the liberal use of pacemakers for conduction disorders observed in our series of TAVI patients. This liberal approach may be debatable, but in elderly patients with several comorbidities preventive pacemaker insertion is justified by guideline recommendation [25]. Piazza et al. [26, 27] showed that some of the initial conduction delay after TAVI was partially reversible at 1-month follow-up and presumably related to inflammation and edema around the conduction pathways [26, 27]; in our series we could not identify a single case of conduction recovery.
Notably, we demonstrated a higher pacemaker requirement rate than seen after open surgical technique. A critical issue, however, is that, with surgical replacement, the native valve is explanted, whereas with TAVI, the native aortic valve remains in situ and is compressed by stent frame against the surrounding structures. Other potential sources of local damage can include degeneration and calcification of the conduction system, the mechanical or ischemic effects of pre-implantation balloon valvuloplasty, or the direct contact and trauma by catheters and guidewires with components of the conduction system; however, trauma from percutaneous balloon aortic valvuloplasty is often reversible. The close anatomic vicinity of the His bundle to the non-coronary and right-coronary cusp results in a mechanical damage of the compact AV node and bundle of His, predominantly (but not exclusively) of the infra-His region during TAVI. Additionally, compression from nitinol expansion (over hours and days) explains further mechanical trauma and “late” onset of conduction disturbances. Actually we have no data according to the time course of prosthesis expansion. Our experience showed that most of the conduction disturbances emerged within the first 7 days.
Nevertheless, with the balloon-expandable shorter Edwards SAPIEN prosthesis, which is placed in the aortic annulus without direct impact on left ventricular outflow tract (LVOT), the incidence of AV conduction block requiring pacemaker was reported between 0 and 6% and new onset LBBB of 3.3% [28, 29].
Although yet to be investigated, technical strategies to diminish trauma to the conduction system by TAVI using the CoreValve revalving system may reduce the risk of conduction abnormalities. Such strategies may include limiting the depth of the valve within the LVOT and keeping the number of pre- and post-valve implantation balloon valvuloplasties to a minimum. Additionally, operators should deploy the device only a few millimeters below the annulus and avoid impacting the septum. A modified implantation technique, however, may also require technical modifications to avoid malalignment of valve. Such new challenging technical approach was, however, not utilized in our early implantation experience.
Conclusion
Cardiac conduction disturbances were common after TAVI and need close surveillance at least during the first week after implantation. The indication for pacemakers in our patient population was liberal and somewhat prophylactic due to the combination of new LBBB with an increase in AV conduction time, especially HV interval. Further evaluation with long-term follow-up is required to analyze a potential temporal evolution of these conduction disturbances as well as progression of the combination of new LBBB with an increase of AV conduction time to complete heart block.
References
Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M (2006) Burden of valvular heart diseases: a population-based study. Lancet 368(9540):1005–1011
Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A (2007) Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology; ESC Committee for Practice Guidelines Guidelines on the management on valvular heart disease of the European Society of Cardiology. Eur Heart J 28:230–268
Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, American College of Cardiology/American Heart Association Task Force on Practice Guidelines (2008) ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Society of Cardiovascular Anaesthesiologists: endorsed by the Society for Cardiovascular Angiography and interventions and society of thoracic surgeons. Circulation 52:e1–e142
Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A (2003) A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 24:1231–1243
Varadarajan P, Kapoor N, Bansal RC, Pai RG (2006) Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 82:2111–2115
Shareghi S, Rasouli L, Shavelle DM, Burstein S, Matthews RV (2007) Current results of balloon aortic valvuloplasty in high-risk patients. J Invasive Cardiol 19:1–5
Sack S, Kahlert P, Khandanpour S, Naber C, Philip S, Möhlenkamp S, Sievers B, Kälsch H, Erbel R (2008) Revival of an old method with new techniques: balloon aortic valvuloplasty of the calcified aortic stenosis in the elderly. Clin Res Cardiol 97:288–297
El-Khally Z, Thibault B, Stainloae C, Theroux P, Dubuc M, Roy D, Guerra P, Macle L, Talajic M (2004) Prognostic significance of newly acquired bundle branch block after aortic valve replacement. Am J Cardiol 94:1008–1011
Marchenese K, Schenk EA (1972) Atrioventricular conduction system lesion following cardiac valve replacement. Circulation 45–46(Suppl. II):II-188
Vahanian A, Alfieri O, Al-Attar N, Antunes M, Bax J, Cormier B, Cribier A, De Jaegere P, Fournial G, Kappetein AP, Kovac J, Ludgate S, Maisano F, Moat N, Mohr F, Nataf P, Pierard L, Pomar JL, Schofer J, Tornos P, Tuzcu M, van Hout B, Von Segesser LK, Walther T (2008) Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Intervention (EAPCI). Eur Heart J 29:1463–1470
Figulla HR, Cremer J, Walther T, Gerckens U, Erbel R, Osterspey A, Zahn R (2009) Positionspapier zur kathetergeführten Aortenklappenintervention. Kardiologe 3:199–206
Zahn R, Gerckens U, Grube E, Linke A, Sievert H, Eggebrecht H, Hambrecht R, Sack S, Hauptmann KE, Richadt G, Figulla HR, Senges J, Investigators German Transcatheter Aortic Valve Intervention-Registry (2011) Transcatheter aortic valve implantation: first results from a multi-centre real-world registry. Eur Heart J 32:198–204
Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW (2006) Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 114:1616–1624
Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, Sauren B, Mohr FW, Walther T, Zickmann B, Iversen S, Felderhoff T, Cartier R, Bonan R (2007) Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 50:69–76
Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S (2006) Percutaneous aortic valve implantation retrograde from the femoral artery. Circulation 113:842–850
Zahn R, Schiele R, Kilkowski C, Klein B, Zeymer U, Werling C, Lehmann A, Layer G, Saggau W (2010) There are two sides to everything: two case reports on sequelae of rescue interventions to treat complications of transcatheter aortic valve implantation of the Medtronic CoreValve prosthesis. Clin Res Cardiol 99:579–585
Zahn R, Schiele R, Kilkowski C, Zeymer U (2010) Severe aortic regurgitation after percutaneous transcatheter aortic valve implantation: on the importance to clarify the underlying pathophysiology. Clin Res Cardiol 99:193–197
Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, Cortina J, David M, Faichney A, Gabrielle F, Gams E, Harjula A, Jones MT, Pintor PP, Salamon R, Thulin L (1999) Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 15:816–822
Yater WM, Cornell VH (1935) Heart block due to calcareous lesions of the bundle of His. Ann Int Med 8:777
Ablaza SG, Blanco G, Maranhao V, Morse DP, Nichols HT (1968) Calcific aortic valvular disease associated with complete heart block. Case reports of successful correction. Dis Chest 54:457–460
Schwartz LS, Goldfischer J, Sprague GJ, Schwartz SP (1969) Syncope and sudden death in aortic stenosis. Am J Cardiol 23:647–658
Copeland JG, Griepp RB, Stinson EB, Shumway NE (1977) Long-term follow-up after isolated aortic valve replacement. J Thorac Cardiovasc Surg 74:875–889
Marcheix B, Lamarche Y, Berry C, Asgar A, Laborde JC, Basmadjian A, Ducharme A, Denault A, Bonan R, Cartier R (2007) Surgical aspects of endovascular retrograde implantation of the aortic CoreValve bioprosthesis in high-risk older patients with severe symptomatic aortic stenosis. J Thorac Cardiovasc Surg 134:1150–1156
Scheinman MM, Peters RW, Modin G, Brennan M, Mies C, O’Young J (1977) Prognostic value of infranodal conduction time in patients with chronic bundle branch block. Circulation 56:240–244
Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW (2008) American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE Guideline. Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons (2008) ACC/AHA/HRS 2008 Guidelines dor Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol 51:e1–e62
Piazza N, Onuma Y, Jesserun E, Kint PP, Maugenest AM, Anderson RH, de Jaegere PP, Serruys PW (2008) Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve. JACC Cardiovasc Interv 1:310–316
Piazza N, Nuis RJ, Tzikas A, Otten A, Onuma Y, García-García H, Schultz C, van Domburg R, van Es GA, van Geuns R, de Jaegere P, Serruys PW (2010) Persistent conduction abnormalities and requirements for pacemaking six months after transcatheter aortic valve implantation. EuroIntervention 6:475–484
Sinhal A, Altwegg L, Pasupati S, Humphries KH, Allard M, Martin P, Cheung A, Ye J, Kerr C, Lichtenstein SV, Webb JG (2008) Atrioventricular block after transcatheter balloon expandable aortic valve implantation. J Am Coll Cardiol Intv 1:305–309
Walther T, Falk V, Kempfert J, Borger MA, Fassl J, Chu MW, Schuler G, Mohr FW (2008) Transapical minimally invasive aortic valve implantation; the initial 50 patients. Eur J Cardiothorac Surg 33:983–988
Conflict of interest
No conflict of interest for IA, SK, HS, AL, JO, DB, TCR, OT, RS, GK, HK, TC, CAN.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
I. Akin and S. Kische contributed equally.
Dr. Ince is an accredited proctor to Medtronic CoreValve system.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Akin, I., Kische, S., Schneider, H. et al. Surface and intracardiac ECG for discriminating conduction disorders after CoreValve implantation. Clin Res Cardiol 101, 357–364 (2012). https://doi.org/10.1007/s00392-011-0400-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-011-0400-6