Abstract
Objective
A recently developed immunoassay for high-sensitivity measurement of cardiac troponin T (hsTnT) allows measurement at the 99th percentile for a normal population with an assay imprecision <10%. It is unclear whether such a low cutpoint (14 ng/L) is helpful for long-term risk stratification of patients with an acute coronary syndrome (ACS) undergoing routine early invasive strategy.
Patients and main outcome measures
Consecutive patients with ACS admitted to a chest pain unit were studied. The usefulness of hsTnT for early diagnosis of myocardial infarction (MI) and prediction of all-cause death or death/MI over a median of 271 days following presentation was compared against the fourth generation cTnT at the 99th percentile cutpoint.
Results
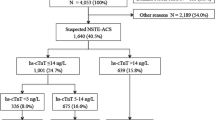
Of 1,384 patients with ACS enrolled, 47.8% had non-ST-segment elevation MI (NSTEMI), 26.4% unstable angina, 21.8% STEMI and 4% had non-ACS. Adjusted risk for all-cause death [adjusted HR 8.26 (95%CI: 1.13–66.33), p = 0.038] and death/MI [adjusted HR 2.71 (95% CI: 1.15–6.38), p = 0.023] were significantly higher with hsTnT above the 99th percentile. In particular, among patients with a standard fourth generation cTnT result below the 99th percentile cutoff (0.01 ng/mL), hsTnT improved risk assessment. Mortality risk associated with an elevated hsTnT was present across the spectrum of ACS, as well as in conditions with hsTnT elevations not related to ACS.
Conclusion
hsTnT at the 99th percentile cutoff is useful for the diagnostic evaluation of patients with ACS, and provides strong and independent predictive power for adverse long-term outcomes even after early invasive strategy.


Similar content being viewed by others
References
Morrow DA, Cannon CP, Jesse RL et al (2007) Academy of Clinical Biochemistry laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem 53:552–574
Bassand JP, Hamm CW, Ardissino D et al (2007) Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. The Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur Heart J 28:1598–1660
Katus HA, Giannitsis E, Jaffe AS et al (2009) Higher sensitivity troponin assays: Quo vadis? Eur Heart J 30:127–128
Mingels A, Jacobs L, Michielsen E et al (2009) Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem 55:101–108
Melanson SE, Morrow DA, Jarolim P (2007) Earlier detection of myocardial injury in a preliminary evaluation using a new troponin I assay with improved sensitivity. Am J Clin Pathol 128:282–286
Apple FS, Pearce LA, Smith SW et al (2009) Role of monitoring changes in sensitive cardiac troponin I assay results for early diagnosis of myocardial infarction and prediction of risk of adverse events. Clin Chem 55:930–937
Zethelius B, Johnston N, Venge P (2006) Troponin I as a predictor of coronary heart disease and mortality in 70-year-old men: a community-based cohort study. Circulation. 113:1071
Eggers KM, Lind L, Ahlström H et al (2008) Prevalence and pathophysiological mechanisms of elevated cardiac troponin I levels in a population-based sample of elderly subjects. Eur Heart J 29:2252–2258
Latini R, Masson S, Anand IS et al (2007) Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 116:1242–1249
Schulz O, Kirpal K, Stein J et al (2006) Importance of low concentrations of cardiac troponins. Clin Chem 52:1614–1615
Lankeit M, Dellas C, Katus HA et al (2009) Additive prognostische Information durch die Verwendung eines hoch sensitiven Assays für Troponin T bei Patienten mit akuter Lungenembolie. Clin Res Cardiol; 98 (Suppl 1):P 1015 abstract
Keller T, Zeller T, Peetz D et al (2009) Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med 361:868–877
Reichlin T, Hochholzer W, Bassetti S et al (2009) Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 361:858–867
Kurz K, Giannitsis E, Becker M et al (2011) Comparison of the new high sensitive cardiac troponin T with myoglobin, h-FABP and cTnT for early identification of myocardial necrosis in the acute coronary syndrome. Clin Res Cardiol 100:209–215
Apple FS, Smith SW, Pearce LA et al (2008) Use of the bioMérieux VIDAS troponin I ultra assay for the diagnosis of myocardial infarction and detection of adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chim Acta 390:72–75
Apple FS, Smith SW, Pearce LA et al (2008) Use of the Centaur TnI-Ultra assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 54:723–728
Kavsak PA, Newman AM, Lustig V et al (2007) Long-term health outcomes associated with detectable troponin I concentrations. Clin Chem 53:220–227
Neizel M, Steen H, Korosoglou G et al (2009) Minor troponin T elevation in patients 6 months after acute myocardial infarction: an observational study. Clin Res Cardiol 98:297–304
Breuckmann F, Post F, Giannitsis E, For the Task Force of Chest Pain Units et al (2008) Criteria of the German Cardiac Society—cardiovascular research for Chest Pain. Kardiologe 2:389–394
Thygesen K, Alpert JS, White HD, On behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the redefinition of myocardial infarction (2007) Universal definition of myocardial infarction. Eur Heart J 28:2525–2538
Giannitsis E, Kurz K, Hallermayer K et al (2010) The new high sensitive cardiac troponin T assay. Clin Chem 56:254–256
Kurz K, Giannitsis E, Zehelein J et al (2008) Highly sensitive cardiac troponin T values remain constant after brief exercise- or pharmacologic-induced reversible myocardial ischemia. Clin Chem 54:1234–1238
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr et al (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27:157–172
Eggers KM, Lagerqvist B, Venge P et al (2009) Prognostic value of biomarkers during and after non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 54:357–364
Giannitsis E, Steen H, Kurz K et al (2008) Cardiac magnetic resonance imaging study for quantification of infarct size comparing directly serial versus single time-point measurements of cardiac troponin T. J Am Coll Cardiol 51:307–314
Vasile VC, Babuin L, Giannitsis E et al (2008) Relationship of MRI-determined infarct size and cTnI measurements in patients with ST-elevation myocardial infarction. Clin Chem 54:617–619
Wu AH, Jaffe AS, Apple FS et al (2007) National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: use of cardiac troponin and B-type natriuretic peptide and N-terminal pro B-type natriuretic peptide for etiologies other than acute coronary syndrome and heart failure. Clin Chem 53:2086–2096
Hamm CW, Giannitsis E, Katus HA (2002) Cardiac troponin elevations in patients without acute coronary syndrome. Circulation 106:2871–2872
Apple FS, Smith SW, Pearce LA et al (2008) Use of the Centaur TnI-Ultra assay for detection of myocardial infarction and adverse events in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem 54:723–728
Morrow DA, Cannon CP, Rifai N, TACTICS-TIMI 18 Investigators et al (2001) Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction: results from a randomized trial. JAMA 286:2405–2412
Kastrati A, Mehilli J, Neumann FJ et al (2006) Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA 295:1531–1538
Giannitsis E, Becker M, Kurz K et al (2010) High-sensitivity cardiac Troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem 56:642–650
Morrow DA, Cannon CP, Rifai N, For the TIMI 18 Investigators et al (2002) Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non-ST elevation myocardial infarction: results from a randomized trial. JAMA 286:2405–2412
Acknowledgments
We thank Mr. Oliver Hartmann, a biostatistician from BRAHMS Biomarkers (clinical diagnostics division of Thermo Fisher Scientific) for support regarding statistical analyses. EG has received financial support for clinical trials from Roche Diagnostics, Germany. He is consultant to Roche Diagnostics and receives honoraria for lectures from Roche Diagnostics. JLJ has received grant support from Roche Diagnostics, and has received honoraria for lectures from Roche Diagnostics. HAK has developed the cTnT assay and holds a patent jointly with Roche Diagnostics. He has received grants and research support from several companies, and has received honoraria for lectures from Roche Diagnostics. DZ and GH are employees of Roche Diagnostics responsible for the preclinical testing of hsTnT. The investigations were supported by Roche Diagnostics, Germany providing assays for hsTnT assay.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
392_2011_344_MOESM4_ESM.ppt
Supplemental Fig. 1. Kaplan–Meier survival (A) and event-free survival (B, death/MI) according to four ranges of cTnT (< 0.01 ng/mL, 0.01 ng/mL - < 0.03 ng/mL, ≥ 0.03 ng/mL - < 0.1 ng/mL, and ≥ 0.1 ng/mL). The concentration of 0.01 ng/mL corresponds to the 99th percentile value, 0.03 ng/mL corresponds to the cutoff that can be measured with ≤ 10% CV, and 0.1 ng/mL corresponds to ROC determined cutoff. (PPT 364 kb)
392_2011_344_MOESM5_ESM.ppt
Supplemental Fig. 4. The absolute risk of death and the composite of death/MI in the reference category was 0.2% and 1.4%, respectively. Unadjusted HRs and 95% CIs for all-cause death and the composite of all-cause death or MI by deciles of hsTnT at baseline. The concentration range for hsTnT, rate of death [triangles], or death/MI [circles]) are reported for each decile of hsTnT. HRs and 95% CIs are reported on a logarithmic scale. The HRs (95% CI) for all-cause death (OR 12.94 (1.66-100.98), p = 0.015) and the composite of all-cause death or MI (OR 12.79 (2.94-55.53), p = 0.0007) were significantly higher at decile 4 (median 34.6 ng/L; concentration range 22.5-46.2 ng/L) and higher deciles than for the reference category (decile 1; Fig. 4). Number of patients per decile = 138 to 139. (PPT 119 kb)
Rights and permissions
About this article
Cite this article
Celik, S., Giannitsis, E., Wollert, K.C. et al. Cardiac troponin T concentrations above the 99th percentile value as measured by a new high-sensitivity assay predict long-term prognosis in patients with acute coronary syndromes undergoing routine early invasive strategy. Clin Res Cardiol 100, 1077–1085 (2011). https://doi.org/10.1007/s00392-011-0344-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-011-0344-x