Abstract
Background
Levosimendan is a promising new inodilator agent but its effectiveness in peripartum cardiomyopathy (PPCM) has not been tested in a clinical trial. The authors sought to evaluate the effect of levosimendan therapy and to determine the predictors of clinical outcome in patients with PPCM.
Methods and results
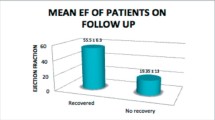
The authors prospectively randomized 24 consecutive women with PPCM. Twelve patients (control group) were randomized to conventional heart failure therapy and 12 patients (levosimendan group) were randomized to levosimendan in addition to the conventional therapy. Mean follow-up period was 20.9 ± 9 months (ranged 12–38 months). The two groups did not differ in baseline demographic and echocardiographic characteristics. Eleven patients (45.8%) recovered completely (6 in control group and 5 in levosimendan group, p > 0.05), 6 died (25%) (3 in control group and 3 in levosimendan group), and 7 (29.1%) were left with persistent left ventricular dysfunction (PLVD) (3 in control group and 4 in levosimendan group, p > 0.05). There were significant differences in baseline characteristics between deceased patients and survivors including left ventricular end-diastolic diameter (7.1 ± 0.6 vs. 6.4 ± 0.5 cm, p = 0.031), left ventricular end-systolic diameter (LVESD) (6.4 ± 0.8 vs. 5.5 ± 0.6 cm, p = 0.027), left ventricular ejection fraction (LVEF) (19.7 vs. 27.4%, p = 0.025), and left atrial diameter (4.9 ± 0.3 vs. 4.3 ± 0.4 cm, p = 0.011).
Conclusions
Addition of levosimendan to conventional therapy did not improve outcome in patients with PPCM. In patients with PLVD or patients who died, LVEF, LVESD and left atrial diameter were worse than those with complete resolution.

Similar content being viewed by others
Abbreviations
- PPCM:
-
Peripartum cardiomyopathy
- NYHA FC:
-
New York heart association functional class
- LVEF:
-
Left ventricular ejection fraction
- PLVD:
-
Persistent left ventricular dysfunction
- LVESD:
-
Left ventricular end-systolic diameter
- LVEDD:
-
Left ventricular end-diastolic diameter
References
Demakis JG, Rahimtoola SH (1971) Peripartum cardiomyopathy. Circulation 44:964–968
Sliwa K, Mocumbi AO (2010) Forgetten cardiovascular diseases in Africa. Clin Res Cardiol 99:65–74
Pearson GD, Veille JC, Rahimtoola S et al (2000) Peripartum cardiomyopathy: National heart, lung and blood institute and office of rare diseases (national institutes of health) workshop recommendations and review. JAMA 283:1088–1183
Hilfiker-Kleiner D, Kaminski K, Podewski E et al (2007) A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128:589–600
Lehmann A, Boldt J, Kirchner J (2003) The role of Ca-sensitizers for the treatment of heart failure. Curr Opin Crit Care 9:337–344
Hitz MP, Bertram H, Köditz H (2008) Levosimendan for bridging in a pediatric patient with Alström syndrome awaiting heart-lung transplantation. Clin Res Cardiol 97:846–848
Follath F, Cleland JG, Just H et al (2002) Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 360:196–202
Moiseyev VS, Poder P, Andrejevs N, Ruda MY, RUSSLAN Study Investigators (2002) Safety and efficacy of a novel calcium sensitizer, levosimendan, in patients with left ventricular failure due to an acute myocardial infarction. A randomized, placebo-controlled, double-blind study (RUSSLAN). Eur Heart J 23:1422–1432
Mebazaa A, Nieminen MS, Packer M, SURVIVE Investigators et al (2007) Levosimendan vs. dobutamine for patients with acute decompensated heart failure: the SURVIVE randomized trial. JAMA 297:1883–1891
De Luca L, Colucci WS, Nieminen MS, Massie BM, Gheorghiade M (2006) Evidence-based use of levosimendan in different clinical settings. Eur Heart J 27(16):1908–1920
Benlolo S, Lefoll C, Katchatouryan V, Payen D, Mebazaa A (2004) Successful use of levosimendan in a patient with peripartum cardiomyopathy. Anesth Analg 98:822–824
Nguyen HD, McKeown B (2005) Levosimendan for post-partum cardiomyopathy. Crit Care Resusc 7:107–110
Benezet-Mazuecos J, de la Hera J (2008) Peripartum cardiomyopathy: a new successful setting for levosimendan. Int J Cardiol 123(3):346–347
Gottdiener JS, Bednarz J, Devereux R, American Society of Echocardiography et al (2004) American society of echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr 17:1086–1119
Midei MG, DeMent SH, Feldman AM, Hutchins GM, Baughman KL (1990) Peripartum myocarditis and cardiomyopathy. Circulation 81:922–928
Bozkurt B, Villaneuva FS, Holubkov R et al (1999) Intravenous immune globulin in the therapy of peripartum cardiomyopathy. J Am Coll Cardiol 34:177–180
Biteker M, Duran NE, Ozkan M (2009) The role of bromocriptine in peripartum cardiomyopathy. Am J Obstet Gynecol 201(2):e13
Sliwa K, Blauwet L, Tibazarwa K et al (2010) Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy. A proof-of-concept pilot study. Circulation 121(13):1465–1473
Sliwa K, Hilfiker-Kleiner D, Petrie MC et al (2010) Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the heart failure association of the European society of cardiology working group on peripartum cardiomyopathy. Eur J Heart Fail 12(8):767–778
Duran N, Günes H, Duran I, Biteker M, Ozkan M (2008) Predictors of prognosis in patients with peripartum cardiomyopathy. Int J Gynaecol Obstet 101:137–140
O’Connell JB, Costanzo-Nordin MR, Subramanian R et al (1986) Peripartum cardiomyopathy: clinical, hemodynamic, histologic and prognostic characteristics. J Am Coll Cardiol 8:52–56
Amos AM, Jaber WA, Russell SD (2006) Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J 152:509–513
Acknowledgments
The authors would like to thank Biykem Bozkurt for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biteker, M., Duran, N.E., Kaya, H. et al. Effect of levosimendan and predictors of recovery in patients with peripartum cardiomyopathy, a randomized clinical trial. Clin Res Cardiol 100, 571–577 (2011). https://doi.org/10.1007/s00392-010-0279-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-010-0279-7